Adrenomedullin and its receptors are expressed in mouse pancreatic β-cells and suppresses insulin synthesis and secretion
- PMID: 35324977
- PMCID: PMC8947024
- DOI: 10.1371/journal.pone.0265890
Adrenomedullin and its receptors are expressed in mouse pancreatic β-cells and suppresses insulin synthesis and secretion
Abstract
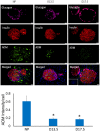
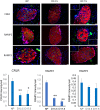
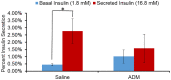
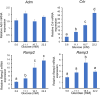
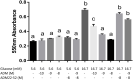
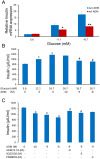
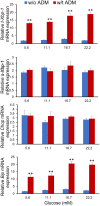
Gestational diabetes mellitus (GDM) is associated with defective pancreatic β-cell adaptation in pregnancy, but the underlying mechanism remains obscure. Our previous studies demonstrated that GDM women display increased plasma adrenomedullin (ADM) levels, and non-obese GDM mice show decreased serum concentrations of insulin and the number of β-cells in pancreas islets. The aims of this study is to examine if ADM and its receptors are expressed in female mouse pancreas, and if so, whether insulin secretion is regulated by ADM in mouse β-cell line, NIT-1 cells and isolated mouse pancreatic islets. Present study shows that ADM and its receptor components CRLR, RAMPs are present in mouse pancreatic islets and co-localized with insulin. The expressions of ADM, CRLR and RAMP2 in islets from pregnant mice are reduced compared to that of non-pregnant mice. NIT-1-β cells express ADM and its receptor mRNA, and glucose dose-dependently stimulates expressions. Furthermore, ADM inhibits NIT-1-β cell growth, and this inhibition is reversed by ADM antagonist, ADM22-52. The glucose-induced insulin secretion was suppressed by ADM in NIT-1-β cells and isolated pancreatic islets from pregnant mice. These inhibitory effects are accompanied by upregulation of endoplasmic reticulum (ER) stress biomarker genes in NIT-1-β cells. This study unveils that reduced ADM and its receptors may play a role in β-cell adaptation during pregnancy, while increased plasma ADM in GDM may contribute to the β-cells dysfunction, and blockade of ADM may reverse β-cell insulin production.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Circulating Adrenomedullin Is Elevated in Gestational Diabetes and Its Role in Impaired Insulin Production by β-Cells.J Clin Endocrinol Metab. 2019 Mar 1;104(3):697-706. doi: 10.1210/jc.2018-01119. J Clin Endocrinol Metab. 2019. PMID: 30383252 Free PMC article.
-
Adrenomedullin has a cytoprotective role against endoplasmic reticulum stress for pancreatic β-cells in autocrine and paracrine manners.J Diabetes Investig. 2020 Jul;11(4):823-833. doi: 10.1111/jdi.13218. Epub 2020 Mar 10. J Diabetes Investig. 2020. PMID: 31989791 Free PMC article.
-
Lipid dysfunction and adrenomedullin expression in omental versus subcutaneous adipose tissues in diabetic pregnancies.PLoS One. 2022 Apr 7;17(4):e0265419. doi: 10.1371/journal.pone.0265419. eCollection 2022. PLoS One. 2022. PMID: 35390031 Free PMC article.
-
Regulation of pancreatic physiology by adrenomedullin and its binding protein.Regul Pept. 2003 Apr 15;112(1-3):121-30. doi: 10.1016/s0167-0115(03)00030-2. Regul Pept. 2003. PMID: 12667633 Review.
-
β-Cell adaptation in pregnancy.Diabetes Obes Metab. 2016 Sep;18 Suppl 1(Suppl 1):63-70. doi: 10.1111/dom.12716. Diabetes Obes Metab. 2016. PMID: 27615133 Free PMC article. Review.
Cited by
-
HAMSAB diet ameliorates dysfunctional signaling in pancreatic islets in autoimmune diabetes.iScience. 2023 Dec 10;27(1):108694. doi: 10.1016/j.isci.2023.108694. eCollection 2024 Jan 19. iScience. 2023. PMID: 38213620 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

