Supplementation with dietary omega-3 PUFA mitigates fetal brain inflammation and mitochondrial damage caused by high doses of sodium nitrite in maternal rats
- PMID: 35324981
- PMCID: PMC8947126
- DOI: 10.1371/journal.pone.0266084
Supplementation with dietary omega-3 PUFA mitigates fetal brain inflammation and mitochondrial damage caused by high doses of sodium nitrite in maternal rats
Abstract
Objective: Food safety and nutrition during pregnancy are important concerns related to fetal brain development. In the present study, we aimed to explore the effects of omega-3 polyunsaturated fatty acids (PUFA ω-3) on exogenous sodium nitrite intervention-induced fetal brain injury in pregnant rats.
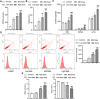
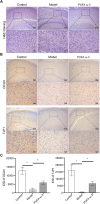
Methods: During pregnancy, rats were exposed to water containing sodium nitrite (0.05%, 0.15%, and 0.25%) to establish a fetal rat brain injury model. Inflammatory factors and oxidative stress levels were detected using enzyme-linked immunosorbent assay (ELISA) or flow cytometry. Subsequently, animals were divided into three groups: control, model, and 4% PUFA ω-3. Pregnancy outcomes were measured and recorded. Hematoxylin-eosin (H&E) staining and immunohistochemistry (IHC) were utilized to observe brain injury. ELISA, quantitative real-time PCR (qRT-PCR), western blot, flow cytometry, and transmission electron microscopy (TEM) were adopted to measure the levels of inflammatory factors, the NRF1/HMOX1 signaling pathway, and mitochondrial and oxidative stress damage.
Results: With the increase of sodium nitrite concentration, the inflammatory factors and oxidative stress levels increased. Therefore, the high dose group was set as the model group for the following experiments. After PUFA ω-3 treatment, the fetal survival ratio, average body weight, and brain weight were elevated. The cells in the PUFA ω-3 group were more closely arranged and more round than the model. PUFA ω-3 treatment relieved inflammatory factors, oxidative stress levels, and mitochondria damage while increasing the indicators related to brain injury and NRF1/HMOX1 levels.
Conclusions: Sodium nitrite exposure during pregnancy could cause brain damage in fetal rats. PUFA ω-3 might help alleviate brain inflammation, oxidative stress, and mitochondrial damage, possibly through the NRF1/HMOX1 signaling pathway. In conclusion, appropriately reducing sodium nitrite exposure and increasing PUFA omega-3 intake during pregnancy may benefit fetal brain development. These findings could further our understanding of nutrition and health during pregnancy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury.J Neuroinflammation. 2017 Jul 24;14(1):143. doi: 10.1186/s12974-017-0917-3. J Neuroinflammation. 2017. PMID: 28738820 Free PMC article.
-
Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury.J Neuroinflammation. 2018 Apr 20;15(1):116. doi: 10.1186/s12974-018-1151-3. J Neuroinflammation. 2018. PMID: 29678169 Free PMC article.
-
Maternal dietary omega-3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat.Biol Reprod. 2013 Feb 14;88(2):37. doi: 10.1095/biolreprod.112.103754. Print 2013 Feb. Biol Reprod. 2013. PMID: 23269667
-
Role of omega-3 polyunsaturated fatty acids in gestational diabetes, maternal and fetal insights: current use and future directions.J Matern Fetal Neonatal Med. 2021 Jan;34(1):124-136. doi: 10.1080/14767058.2019.1593361. Epub 2019 Mar 27. J Matern Fetal Neonatal Med. 2021. PMID: 30857450 Review.
-
Maternal dietary omega-3 fatty acids and placental function.Reproduction. 2014 Apr 8;147(5):R143-52. doi: 10.1530/REP-13-0376. Print 2014 May. Reproduction. 2014. PMID: 24451224 Review.
Cited by
-
Safety and efficacy of antioxidant therapy in children and adolescents with attention deficit hyperactivity disorder: A systematic review and network meta-analysis.PLoS One. 2024 Mar 28;19(3):e0296926. doi: 10.1371/journal.pone.0296926. eCollection 2024. PLoS One. 2024. PMID: 38547138 Free PMC article.
References
-
- Lindsay KL, Buss C, Wadhwa PD, Entringer S. The Interplay Between Nutrition and Stress in Pregnancy: Implications for Fetal Programming of Brain Development. Biol Psychiatry. 2019;85(2):135–49. Epub 2018/07/31. doi: 10.1016/j.biopsych.2018.06.021 ; PubMed Central PMCID: PMC6389360. - DOI - PMC - PubMed
-
- Franco PN, Durrant LM, Doan C, Carreon D, Beltran A, Jullienne A, et al.. Maternal Undernutrition Modulates Neonatal Rat Cerebrovascular Structure, Function, and Vulnerability to Mild Hypoxic-Ischemic Injury via Corticosteroid-Dependent and -Independent Mechanisms. Int J Mol Sci. 2021;22(2). Epub 2021/01/16. doi: 10.3390/ijms22020680 ; PubMed Central PMCID: PMC7827870. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

