Polymorphisms in the hypoxia inducible factor binding site of the macrophage migration inhibitory factor gene promoter in schizophrenia
- PMID: 35324982
- PMCID: PMC8946738
- DOI: 10.1371/journal.pone.0265738
Polymorphisms in the hypoxia inducible factor binding site of the macrophage migration inhibitory factor gene promoter in schizophrenia
Abstract
Background: Macrophage migration inhibitory factor (MIF) is a multifunctional cytokine that promotes neurogenesis and neuroprotection. MIF is predominantly expressed in astrocytes in the brain. The serum MIF level and microsatellites/single nucleotide polymorphisms (SNPs) in the MIF gene promoter region are known to be associated with schizophrenia (SCZ). Interestingly, previous studies reported that hypoxia, an environmental risk factor for SCZ, induced MIF expression through binding of the hypoxia inducible factor (HIF)-1 to the hypoxia response element (HRE) in the MIF promoter.
Methods: We investigated the involvement of MIF in SCZ while focusing on the HIF pathway. First, we conducted an association study of the SNP rs17004038 (C>A) in the HRE of the MIF promoter between 1758 patients with SCZ and 1507 controls. Next, we investigated the effect of hypoxia on MIF expression in primary cultured astrocytes derived from neonatal mice forebrain.
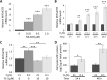
Results: SNP rs17004038 was significantly associated with SCZ (p = 0.0424, odds ratio = 1.445), indicating that this SNP in the HRE of the MIF promoter was a genetic risk factor for SCZ. Hypoxia induced MIF mRNA expression and MIF protein production and increased HIF-1 binding to the MIF promoter, while the activity of the MIF promoter was suppressed by mutations in the HRE and by deletion of the HRE in astrocytes.
Conclusion: These results suggest that SNP rs17004038 in the HRE of the MIF promoter was significantly associated with SCZ and may be involved in the pathophysiology of SCZ via suppression of hypoxia and HIF pathway-induced MIF expression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Cattane N, Richetto J, Cattaneo A. Prenatal exposure to environmental insults and enhanced risk of developing Schizophrenia and Autism Spectrum Disorder: focus on biological pathways and epigenetic mechanisms. Neurosci Biobehav Rev. 2020;117:253–78. doi: 10.1016/j.neubiorev.2018.07.001 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

