Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: from exosomes to microvesicles
- PMID: 35325061
- PMCID: PMC10233250
- DOI: 10.1093/cvr/cvac031
Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: from exosomes to microvesicles
Abstract
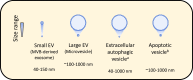
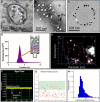
Extracellular vesicles (EVs) are nanosized vesicles with a lipid bilayer that are released from cells of the cardiovascular system, and are considered important mediators of intercellular and extracellular communications. Two types of EVs of particular interest are exosomes and microvesicles, which have been identified in all tissue and body fluids and carry a variety of molecules including RNAs, proteins, and lipids. EVs have potential for use in the diagnosis and prognosis of cardiovascular diseases and as new therapeutic agents, particularly in the setting of myocardial infarction and heart failure. Despite their promise, technical challenges related to their small size make it challenging to accurately identify and characterize them, and to study EV-mediated processes. Here, we aim to provide the reader with an overview of the techniques and technologies available for the separation and characterization of EVs from different sources. Methods for determining the protein, RNA, and lipid content of EVs are discussed. The aim of this document is to provide guidance on critical methodological issues and highlight key points for consideration for the investigation of EVs in cardiovascular studies.
Keywords: Biodistribution; Blood; Cardiovascular diseases; Exosomes; Extracellular vesicle composition; Heart; Microvesicles; Therapeutics.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflicts of interest: L.B. has performed advisory board work and received speaker fees from Sanofi and Novartis, and is founder and shareholder of Glycardial Diagnosis SL and Ivestatin Therapeutics, SL (all outside of this work); C.J.B. is a board member of Technoclone. A.B. is the founder and CEO of Exo-Analysis. T.T. has filed and licensed patents in the field of non-coding RNAs and targeted delivery strategies and is the founder and shareholder of Cardior Pharmaceuticals GmbH (outside of the topic of this review). R.L. discloses grants from Stago and a patent on microvesicle fibrinolytic activity licensed to Stago. E.I.B. is a member of the Advisory Board of Sphere Gene Therapeutics Inc. (Boston, USA). M.H.M.W. discloses a collaborative research agreement with BD Biosciences Europe, Erembodegem, Belgium to optimize flow cytometric analysis of EVs. “This manuscript was handled by Reviews Deputy Editor Dr Ali J. Marian”.
Figures

References
-
- Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat ML, Boilard E, Buzas EI, Caporali A, Dignat-George F, Evans PC, Lacroix R, Lutgens E, Ketelhuth DFJ, Nieuwland R, Toti F, Tunon J, Weber C. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost 2017;117:1296–1316. - PubMed
-
- Properzi F, Logozzi M, Fais S. Exosomes: the future of biomarkers in medicine. Biomark Med 2013;7:769–778. - PubMed
-
- Sluijter JPG, Davidson SM, Boulanger CM, Buzás EI, de Kleijn DPV, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers RM, Schulz R, Van Laake LW, Ytrehus K, Ferdinandy P. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2018;114:19–34. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RG/20/9/35101/BHF_/British Heart Foundation/United Kingdom
- PG/18/44/33790/BHF_/British Heart Foundation/United Kingdom
- CH/15/1/31199/BHF_/British Heart Foundation/United Kingdom
- R01 DA047807/DA/NIDA NIH HHS/United States
- R33 MH118164/MH/NIMH NIH HHS/United States
- CH/16/3/32406/BHF_/British Heart Foundation/United Kingdom
- UH3 CA241694/CA/NCI NIH HHS/United States
- RG/F/21/110053/BHF_/British Heart Foundation/United Kingdom
- PG/21/10798/BHF_/British Heart Foundation/United Kingdom
- FS/PHD/22/29237/BHF_/British Heart Foundation/United Kingdom
- R01 AI144997/AI/NIAID NIH HHS/United States

