Endothelial OCT4 is atheroprotective by preventing metabolic and phenotypic dysfunction
- PMID: 35325071
- PMCID: PMC9890633
- DOI: 10.1093/cvr/cvac036
Endothelial OCT4 is atheroprotective by preventing metabolic and phenotypic dysfunction
Abstract
Aims: Until recently, the pluripotency factor Octamer (ATGCAAAT)-binding transcriptional factor 4 (OCT4) was believed to be dispensable in adult somatic cells. However, our recent studies provided clear evidence that OCT4 has a critical atheroprotective role in smooth muscle cells. Here, we asked if OCT4 might play a functional role in regulating endothelial cell (EC) phenotypic modulations in atherosclerosis.
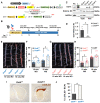
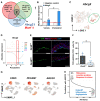
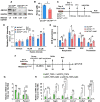
Methods and results: Specifically, we show that EC-specific Oct4 knockout resulted in increased lipid, LGALS3+ cell accumulation, and altered plaque characteristics consistent with decreased plaque stability. A combination of single-cell RNA sequencing and EC-lineage-tracing studies revealed increased EC activation, endothelial-to-mesenchymal transitions, plaque neovascularization, and mitochondrial dysfunction in the absence of OCT4. Furthermore, we show that the adenosine triphosphate (ATP) transporter, ATP-binding cassette (ABC) transporter G2 (ABCG2), is a direct target of OCT4 in EC and establish for the first time that the OCT4/ABCG2 axis maintains EC metabolic homeostasis by regulating intracellular heme accumulation and related reactive oxygen species production, which, in turn, contributes to atherogenesis.
Conclusions: These results provide the first direct evidence that OCT4 has a protective metabolic function in EC and identifies vascular OCT4 and its signalling axis as a potential target for novel therapeutics.
Keywords: ABCG2; Atherosclerosis; Heme; OCT4; ROS.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2022. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared.
Figures





Similar articles
-
Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis.Circulation. 2020 Nov 24;142(21):2045-2059. doi: 10.1161/CIRCULATIONAHA.120.046672. Epub 2020 Jul 17. Circulation. 2020. PMID: 32674599 Free PMC article.
-
Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective.Nat Med. 2016 Jun;22(6):657-65. doi: 10.1038/nm.4109. Epub 2016 May 16. Nat Med. 2016. PMID: 27183216 Free PMC article.
-
Endothelial Cell-Specific Deletion of P2Y2 Receptor Promotes Plaque Stability in Atherosclerosis-Susceptible ApoE-Null Mice.Arterioscler Thromb Vasc Biol. 2017 Jan;37(1):75-83. doi: 10.1161/ATVBAHA.116.308561. Epub 2016 Nov 17. Arterioscler Thromb Vasc Biol. 2017. PMID: 27856454 Free PMC article.
-
Zinc finger E-box binding homeobox 1 and atherosclerosis: New insights and therapeutic potential.J Cell Physiol. 2021 Jun;236(6):4216-4230. doi: 10.1002/jcp.30177. Epub 2020 Dec 4. J Cell Physiol. 2021. PMID: 33275290 Review.
-
Role of PGC-1α in Vascular Regulation: Implications for Atherosclerosis.Arterioscler Thromb Vasc Biol. 2016 Aug;36(8):1467-74. doi: 10.1161/ATVBAHA.116.307123. Epub 2016 Jun 16. Arterioscler Thromb Vasc Biol. 2016. PMID: 27312223 Free PMC article. Review.
Cited by
-
Endothelial-to-Mesenchymal Transition in Atherosclerosis: Friend or Foe?Cells. 2022 Sep 21;11(19):2946. doi: 10.3390/cells11192946. Cells. 2022. PMID: 36230908 Free PMC article. Review.
-
Vascular Inflammatory Diseases and Endothelial Phenotypes.Cells. 2023 Jun 15;12(12):1640. doi: 10.3390/cells12121640. Cells. 2023. PMID: 37371110 Free PMC article. Review.
-
VARIDT 3.0: the phenotypic and regulatory variability of drug transporter.Nucleic Acids Res. 2024 Jan 5;52(D1):D1490-D1502. doi: 10.1093/nar/gkad818. Nucleic Acids Res. 2024. PMID: 37819041 Free PMC article.
-
Endothelial Cell Phenotypic Plasticity in Cardiovascular Physiology and Disease: Mechanisms and Therapeutic Prospects.Am J Hypertens. 2025 Jun 16;38(7):411-421. doi: 10.1093/ajh/hpaf027. Am J Hypertens. 2025. PMID: 39989120 Review.
-
Cis-regulatory Function of the Pou5f1 Gene Promoter in the Mouse MHC Locus.Acta Naturae. 2025 Apr-Jun;17(2):64-74. doi: 10.32607/actanaturae.27596. Acta Naturae. 2025. PMID: 40771854 Free PMC article.
References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS. Heart disease and stroke Statistics—2019 update: a report from the American Heart Association. Circulation 2019:139:e56–e528. - PubMed
-
- Busse R, Fleming I. Vascular endothelium and blood flow. Handb Exp Pharmacol 2006:43–78. - PubMed
-
- Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep 2012;2:1684–1696. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

