Response to leucine in Schizosaccharomyces pombe (fission yeast)
- PMID: 35325114
- PMCID: PMC9041340
- DOI: 10.1093/femsyr/foac020
Response to leucine in Schizosaccharomyces pombe (fission yeast)
Abstract
Leucine (Leu) is a branched-chain, essential amino acid in animals, including humans. Fungi, including the fission yeast Schizosaccharomyces pombe, can biosynthesize Leu, but deletion of any of the genes in this biosynthesis leads to Leu auxotrophy. In this yeast, although a mutation in the Leu biosynthetic pathway, leu1-32, is clearly inconvenient for this species, it has increased its usefulness as a model organism in laboratories worldwide. Leu auxotrophy produces intracellular responses and phenotypes different from those of the prototrophic strains, depending on the growing environment, which necessitates a certain degree of caution in the analysis and interpretation of the experimental results. Under amino acid starvation, the amino acid-auxotrophic yeast induces cellular responses, which are conserved in higher organisms without the ability of synthesizing amino acids. This mini-review focuses on the roles of Leu in S. pombe and discusses biosynthetic pathways, contribution to experimental convenience using a plasmid specific for Leu auxotrophic yeast, signaling pathways, and phenotypes caused by Leu starvation. An accurate understanding of the intracellular responses brought about by Leu auxotrophy can contribute to research in various fields using this model organism and to the understanding of intracellular responses in higher organisms that cannot synthesize Leu.
Keywords: Schizosaccharomyces pombe; leu1-32; TORC1; fission yeast; general amino acid control; leucine.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures

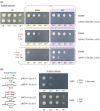

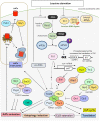
References
-
- Arndt GM, Atkins D. pH sensitivity of Schizosaccharomycespombe: effect on the cellular phenotype associated with lacZ gene expression. Curr Genet. 1996;29:457–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

