Liquid-to-solid phase transition of oskar ribonucleoprotein granules is essential for their function in Drosophila embryonic development
- PMID: 35325593
- PMCID: PMC9042795
- DOI: 10.1016/j.cell.2022.02.022
Liquid-to-solid phase transition of oskar ribonucleoprotein granules is essential for their function in Drosophila embryonic development
Abstract
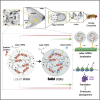
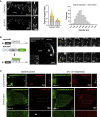
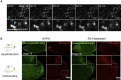
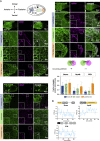
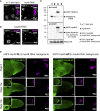
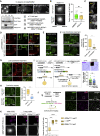
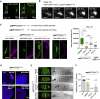
Asymmetric localization of oskar ribonucleoprotein (RNP) granules to the oocyte posterior is crucial for abdominal patterning and germline formation in the Drosophila embryo. We show that oskar RNP granules in the oocyte are condensates with solid-like physical properties. Using purified oskar RNA and scaffold proteins Bruno and Hrp48, we confirm in vitro that oskar granules undergo a liquid-to-solid phase transition. Whereas the liquid phase allows RNA incorporation, the solid phase precludes incorporation of additional RNA while allowing RNA-dependent partitioning of client proteins. Genetic modification of scaffold granule proteins or tethering the intrinsically disordered region of human fused in sarcoma (FUS) to oskar mRNA allowed modulation of granule material properties in vivo. The resulting liquid-like properties impaired oskar localization and translation with severe consequences on embryonic development. Our study reflects how physiological phase transitions shape RNA-protein condensates to regulate the localization and expression of a maternal RNA that instructs germline formation.
Keywords: RNA localization; RNP granules; biomolecular condensates; embryonic development; material properties; oskar mRNA; phase separation; ribonucleoprotein granules; translation control.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures









Comment in
-
oskar stands on solid ground for translation.Nat Rev Mol Cell Biol. 2022 May;23(5):305. doi: 10.1038/s41580-022-00477-8. Nat Rev Mol Cell Biol. 2022. PMID: 35338358 No abstract available.
References
-
- Alberti S. The wisdom of crowds: Regulating cell function through condensed states of living matter. J. Cell Sci. 2017;130:2789–2796. - PubMed
-
- Alberti S., Hyman A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021;22:196–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

