Risks and burdens of incident diabetes in long COVID: a cohort study
- PMID: 35325624
- PMCID: PMC8937253
- DOI: 10.1016/S2213-8587(22)00044-4
Risks and burdens of incident diabetes in long COVID: a cohort study
Abstract
Background: There is growing evidence suggesting that beyond the acute phase of SARS-CoV-2 infection, people with COVID-19 could experience a wide range of post-acute sequelae, including diabetes. However, the risks and burdens of diabetes in the post-acute phase of the disease have not yet been comprehensively characterised. To address this knowledge gap, we aimed to examine the post-acute risk and burden of incident diabetes in people who survived the first 30 days of SARS-CoV-2 infection.
Methods: In this cohort study, we used the national databases of the US Department of Veterans Affairs to build a cohort of 181 280 participants who had a positive COVID-19 test between March 1, 2020, and Sept 30, 2021, and survived the first 30 days of COVID-19; a contemporary control (n=4 118 441) that enrolled participants between March 1, 2020, and Sept 30, 2021; and a historical control (n=4 286 911) that enrolled participants between March 1, 2018, and Sept 30, 2019. Both control groups had no evidence of SARS-CoV-2 infection. Participants in all three comparison groups were free of diabetes before cohort entry and were followed up for a median of 352 days (IQR 245-406). We used inverse probability weighted survival analyses, including predefined and algorithmically selected high dimensional variables, to estimate post-acute COVID-19 risks of incident diabetes, antihyperglycaemic use, and a composite of the two outcomes. We reported two measures of risk: hazard ratio (HR) and burden per 1000 people at 12 months.
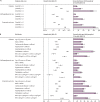
Findings: In the post-acute phase of the disease, compared with the contemporary control group, people with COVID-19 exhibited an increased risk (HR 1·40, 95% CI 1·36-1·44) and excess burden (13·46, 95% CI 12·11-14·84, per 1000 people at 12 months) of incident diabetes; and an increased risk (1·85, 1·78-1·92) and excess burden (12·35, 11·36-13·38) of incident antihyperglycaemic use. Additionally, analyses to estimate the risk of a composite endpoint of incident diabetes or antihyperglycaemic use yielded a HR of 1·46 (95% CI 1·43-1·50) and an excess burden of 18·03 (95% CI 16·59-19·51) per 1000 people at 12 months. Risks and burdens of post-acute outcomes increased in a graded fashion according to the severity of the acute phase of COVID-19 (whether patients were non-hospitalised, hospitalised, or admitted to intensive care). All the results were consistent in analyses using the historical control as the reference category.
Interpretation: In the post-acute phase, we report increased risks and 12-month burdens of incident diabetes and antihyperglycaemic use in people with COVID-19 compared with a contemporary control group of people who were enrolled during the same period and had not contracted SARS-CoV-2, and a historical control group from a pre-pandemic era. Post-acute COVID-19 care should involve identification and management of diabetes.
Funding: US Department of Veterans Affairs and the American Society of Nephrology.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests YX and ZA-A declare support from the US Department of Veterans Affairs for the submitted work. YX declares support for the American Society of Nephrology for the submitted work. ZA-A reports receiving consultation fees from Gilead Sciences and receipt of funding (unrelated to this work) from Tonix Pharmaceuticals. ZA-A is a Member Board of Directors for Veterans Research and Education Foundation of Saint Louis, associate editor for the Journal of the American Society of Nephrology, and is a member of multiple editorial boards.
Figures


Comment in
-
Rising diabetes diagnosis in long COVID.Lancet Diabetes Endocrinol. 2022 May;10(5):298-299. doi: 10.1016/S2213-8587(22)00078-X. Epub 2022 Mar 21. Lancet Diabetes Endocrinol. 2022. PMID: 35325625 Free PMC article. No abstract available.
-
Diabetesrisiko nach COVID-19-Infektion deutlich erhöht.MMW Fortschr Med. 2022 Apr;164(8):16-17. doi: 10.1007/s15006-022-1105-7. MMW Fortschr Med. 2022. PMID: 35449257 Free PMC article. German. No abstract available.
-
Risk of Diabetes Following COVID-19: Translating Evidence Into Clinical and Public Health Actions.J Clin Endocrinol Metab. 2022 Sep 28;107(10):e4248-e4249. doi: 10.1210/clinem/dgac384. J Clin Endocrinol Metab. 2022. PMID: 35749295 Free PMC article. No abstract available.
-
Patients surviving COVID-19 had increased risk for incident diabetes vs. persons without COVID-19.Ann Intern Med. 2022 Aug;175(8):JC93. doi: 10.7326/J22-0052. Epub 2022 Aug 2. Ann Intern Med. 2022. PMID: 35914265
-
COVID-19 erhöht 1-Jahres-Diabetesrisiko.MMW Fortschr Med. 2022 Aug;164(14):28-29. doi: 10.1007/s15006-022-1301-5. MMW Fortschr Med. 2022. PMID: 35941441 Free PMC article. German. No abstract available.
References
-
- Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

