Serial measurement of M. tuberculosis in blood from critically-ill patients with HIV-associated tuberculosis
- PMID: 35325781
- PMCID: PMC8938880
- DOI: 10.1016/j.ebiom.2022.103949
Serial measurement of M. tuberculosis in blood from critically-ill patients with HIV-associated tuberculosis
Abstract
Background: Despite being highly prevalent in hospitalised patients with severe HIV-associated tuberculosis (TB) and sepsis, little is known about the mycobacteriology of Mycobacterium tuberculosis bloodstream infection (MTBBSI). We developed methods to serially measure bacillary load in blood and used these to characterise MTBBSI response to anti-TB therapy (ATT) and relationship with mortality.
Methods: We established a microscopy method for direct visualisation of M. tuberculosis bacilli in blood using a novel lysis-concentration protocol and the fluorescent probe, 4-N,N-dimethylaminonaphthalimide-trehalose (DMN-Tre). We tested blood using GeneXpert® MTB/RIF-Ultra (Xpert-ultra) and Myco/F lytic culture after processing blood through lysis-wash steps to remove PCR inhibitors and anti-microbial drug carry-over. HIV-positive patients predicted to have MTBBSI gave blood samples 0, 4, 24, 48 and 72 h after ATT initiation. Bacillary loads were quantified using microscopy, Xpert-ultra cycle threshold, and culture time-to-positivity. Pharmacodynamics were modelled using these measures combined on an ordinal scale, including association with 12-week mortality.
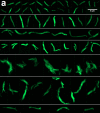
Findings: M. tuberculosis was detected in 27 of 28 recruited participants; 25 (89%) by blood Xpert-ultra, 22 (79%) by DMN-Tre microscopy, and 21 (75%) by Myco/F lytic blood culture. Eight (29%) participants died by 12-week follow-up. In a combined pharmacodynamic model, predicted probabilities of negative DMN-Tre microscopy, blood Xpert-ultra, or blood culture after 72 h treatment were 0·64, 0·27, and 0·94, respectively, in those who survived, compared with 0·23, 0·06, and 0·71 in those who died (posterior probability of slower clearance of MTBBSI in those that died >0·99). DMN-Tre microscopy of blood demonstrated heterogenous bacillary morphologies, including microcolonies and clumps. Bacillary cell-length varied significantly with ATT exposure (mean cell-length increase 0·13 log-µm/day; 95%CrI 0·10-0·16).
Interpretation: Pharmacodynamics of MTBBSI treatment can be captured using DMN-Tre microscopy, blood Xpert-ultra and culture. This could facilitate interventional trials in severe HIV-associated TB.
Funding: Wellcome Trust, NIH Fogarty International Center, South African MRC, NIHR(UK), National Research Foundation of South Africa.
Keywords: Blood stream infection; Critical-illness; HIV-associated tuberculosis; Pharmacodynamics; Sepsis.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests CRB and MK, are cofounders of OliLux Biosciences which has licensed patents related to the Two dyes presented in this paper. All other authors have declared that no conflict of interest exists.
Figures




References
-
- Jacob S.T., Pavlinac P.B., Nakiyingi L., et al. Mycobacterium tuberculosis bacteremia in a cohort of HIV-infected patients hospitalized with severe sepsis in Uganda-high frequency, low clinical suspicion [corrected] and derivation of a clinical prediction score. PLoS One. 2013;8(8):e70305. - PMC - PubMed
-
- Ford N., Shubber Z., Meintjes G., et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2(10):e438–ee44. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

