Genetic Etiologies for Chronic Kidney Disease Revealed through Next-Generation Renal Gene Panel
- PMID: 35325889
- PMCID: PMC9216312
- DOI: 10.1159/000522226
Genetic Etiologies for Chronic Kidney Disease Revealed through Next-Generation Renal Gene Panel
Abstract
Introduction: Chronic kidney disease (CKD) is a major public health issue in the USA. Identification of monogenic causes of CKD, which are present in ∼10% of adult cases, can impact prognosis and patient management. Broad gene panels can provide unbiased testing approaches, which are advantageous in phenotypically heterogeneous diseases. However, the use and yield of broad genetic panels by nephrologists in clinical practice is not yet well characterized.
Methods: Renal genetic testing, ordered exclusively for clinical purposes, predominantly by general and transplant nephrologists within the USA, was performed on 1,007 consecutive unique patient samples. Testing was performed using a commercially available next-generation sequencing-based 382 gene kidney disease panel. Pathogenic (P) and likely pathogenic (LP) variants were reported. Positive findings included a monoallelic P/LP variant in an autosomal dominant or X-linked gene and biallelic P/LP variants in autosomal recessive genes.
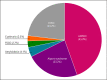
Results: Positive genetic findings were identified in 21.1% (212/1,007) of cases. A total of 220 positive results were identified across 48 genes. Positive results occurred most frequently in the PKD1 (34.1%), COL4A5 (10.9%), PKD2 (10.0%), COL4A4 (6.4%), COL4A3 (5.9%), and TTR (4.1%) genes. Variants identified in the remaining 42 genes comprised 28.6% of the total positive findings, including single positive results in 26 genes. Positive results in >1 gene were identified in 7.5% (16/212) of cases.
Conclusions: Use of broad panel genetic testing by clinical nephrologists had a high success rate, similar to results obtained by academic centers specializing in genetics.
Keywords: Chronic kidney disease; Genetic testing; Nephrology; Next-generation sequencing.
© 2022 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
A.J.B. received speaker fees from Natera and has served on advisory boards for Horizon Therapeutics. J.J.E. received speaker fees from Natera, Otsuka, and Astra Zeneca. M.Z.M. received grant/research support from Viracor and CareDx and served as an advisor for Merck, CareDx, and AbbVie. W.K. is an employee and the owner of Florida Kidney Physicians; has ownership in/is a consultant for DaVita; received speaker fees and honoraria from Natera, Otsuka, Opko, and Astra-Zeneca; received research support from GSK, Tricida, Bayer, Sanifit, DiaMedica, Retrophin, and Astra-Zeneca; and is a member of the advisory boards for Retrophin and Astra-Zeneca. P.M. received support from Natera, Immunocor, CareDX, and Veloxis and is a consultant for CareDx and Natera. Y.X. and S.S. are employees of and own stock in Fulgent Genetics. M.W., J.X., M.S.B., K.B., S.P., Z.P.D., H.T., P.R.B., and T.M. are employees of Natera, Inc. with the option to own stock. F.J. and S.K. have no conflicts of interest to declare.
Figures

References
-
- Centers for Disease Control and Prevention: Chronic Kidney Disease in the United States. 2019. [Available from: https://www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disea...] (accessed March 31, 2021)
-
- Vivante A, Skorecki K. Introducing routine genetic testing for patients with CKD. Nat Rev Nephrol. 2019;15((6)):321–2. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

