Pequi Fruit Extract Increases Antioxidant Enzymes and Reduces Oxidants in Human Coronary Artery Endothelial Cells
- PMID: 35326129
- PMCID: PMC8944551
- DOI: 10.3390/antiox11030474
Pequi Fruit Extract Increases Antioxidant Enzymes and Reduces Oxidants in Human Coronary Artery Endothelial Cells
Abstract
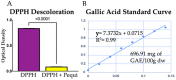
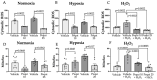
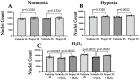
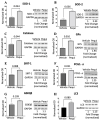
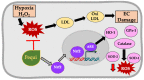
Reactive oxygen species (ROS) imbalance results in endothelial cell function impairment. Natural phenolic antioxidant compounds have been investigated as therapeutic alternatives. The fruit bark of Brazilian-native pequi (Caryocar brasiliense, Camb.) is rich in polyphenols. The HPLC-MS (High-Performance Liquid Chromatography coupled with Mass Spectrometry) analyses identified gallic acid and catechin in six out of seven ethanolic extract samples prepared in our lab. In this study, we examined the effects of ethanolic pequi extract on ROS levels in human coronary artery endothelial cells (HCAEC) subjected to hypoxia or oxidative stress. We first confirmed the oxidant scavenging capacity of the extract. Then, HCAEC pre-incubated with 10 or 25 μg/mL of extract were subjected to hypoxia for 48 h or 100 μM H2O2 for six hours and compared to the normoxia group. Total and mitochondrial ROS levels and cell proliferation were measured. Pequi significantly reduced cytosolic HCAEC ROS levels in all conditions. Mitochondrial ROS were also reduced, except in hypoxia with 10 μg/mL of extract. HCAEC proliferation increased when treated with 25 μg/mL extract under hypoxia and after H2O2 addition. Additionally, pequi upregulated oxidative stress defense enzymes superoxide dismutase (SOD-)1, SOD-2, catalase, and glutathione peroxidase. Together, these findings demonstrate that pequi bark extract increases antioxidative enzyme levels, decreases ROS, and favors HACEC proliferation, pointing to a protective effect against oxidative stress.
Keywords: cardiovascular disease; ethnopharmacology; human coronary artery endothelium; phenols; reactive oxygen species.
Conflict of interest statement
Ruhul Abid is the co-founder of a non-profit organization HAEFA USA (
Figures


Similar articles
-
Tartary Buckwheat Peptides Prevent Oxidative Damage in Differentiated SOL8 Cells via a Mitochondria-Mediated Apoptosis Pathway.Nutrients. 2025 Jul 2;17(13):2204. doi: 10.3390/nu17132204. Nutrients. 2025. PMID: 40647308 Free PMC article.
-
Antioxidant Effects of Moringa oleifera Against Abamectin-Induced Oxidative Stress in the Brain and Erythrocytes of Rats.Chem Biodivers. 2025 May;22(5):e202402709. doi: 10.1002/cbdv.202402709. Epub 2025 Jan 7. Chem Biodivers. 2025. PMID: 39724495
-
[Neuroprotective Effects of Anisodine Hydromide in a Rat Model of Vascular Dementia and the Antioxidative Stress Mechanisms Involved].Sichuan Da Xue Xue Bao Yi Xue Ban. 2025 Mar 20;56(2):324-330. doi: 10.12182/20250360505. Sichuan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40599285 Free PMC article. Chinese.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Phenolic Compounds Characterization of Caryocar brasiliense Peel with Potential Antioxidant Activity.Plants (Basel). 2024 Jul 23;13(15):2016. doi: 10.3390/plants13152016. Plants (Basel). 2024. PMID: 39124134 Free PMC article.
-
Exotic fruits patents trends: An overview based on technological prospection with a focus on Amazonian.Heliyon. 2023 Nov 4;9(12):e22060. doi: 10.1016/j.heliyon.2023.e22060. eCollection 2023 Dec. Heliyon. 2023. PMID: 38046170 Free PMC article.
-
Dysregulation of AR and ERα caused ovarian alterations in gerbils prenatally exposed to 17α-ethinylestradiol and pequi oil.Histochem Cell Biol. 2025 May 22;163(1):57. doi: 10.1007/s00418-025-02389-y. Histochem Cell Biol. 2025. PMID: 40402306
-
Protective Effect of Alpinia oxyphylla Fruit against tert-Butyl Hydroperoxide-Induced Toxicity in HepG2 Cells via Nrf2 Activation and Free Radical Scavenging and Its Active Molecules.Antioxidants (Basel). 2022 May 23;11(5):1032. doi: 10.3390/antiox11051032. Antioxidants (Basel). 2022. PMID: 35624896 Free PMC article.
References
-
- Heron M. Deaths: Leading Causes for 2017. Natl. Vital Stat. Rep. 2019;68:1–76. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

