Human Placental Intracellular Cholesterol Transport: A Focus on Lysosomal and Mitochondrial Dysfunction and Oxidative Stress
- PMID: 35326150
- PMCID: PMC8944475
- DOI: 10.3390/antiox11030500
Human Placental Intracellular Cholesterol Transport: A Focus on Lysosomal and Mitochondrial Dysfunction and Oxidative Stress
Abstract
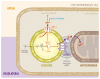
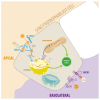
The placenta participates in cholesterol biosynthesis and metabolism and regulates exchange between the maternal and fetal compartments. The fetus has high cholesterol requirements, and it is taken up and synthesized at elevated rates during pregnancy. In placental cells, the major source of cholesterol is the internalization of lipoprotein particles from maternal circulation by mechanisms that are not fully understood. As in hepatocytes, syncytiotrophoblast uptake of lipoprotein cholesterol involves lipoprotein receptors such as low-density lipoprotein receptor (LDLR) and scavenger receptor class B type I (SR-BI). Efflux outside the cells requires proteins such as the ATP-binding cassette (ABC) transporters ABCA1 and ABCG1. However, mechanisms associated with intracellular traffic of cholesterol in syncytiotrophoblasts are mostly unknown. In hepatocytes, uptaken cholesterol is transported to acidic late endosomes (LE) and lysosomes (LY). Proteins such as Niemann-Pick type C 1 (NPC1), NPC2, and StAR related lipid transfer domain containing 3 (STARD3) are required for cholesterol exit from the LE/LY. These proteins transfer cholesterol from the lumen of the LE/LY into the LE/LY-limiting membrane and then export it to the endoplasmic reticulum, mitochondria, or plasma membrane. Although the production, metabolism, and transport of cholesterol in placental cells are well explored, there is little information on the role of proteins related to intracellular cholesterol traffic in placental cells during physiological or pathological pregnancies. Such studies would be relevant for understanding fetal and placental cholesterol management. Oxidative stress, induced by generating excess reactive oxygen species (ROS), plays a critical role in regulating various cellular and biological functions and has emerged as a critical common mechanism after lysosomal and mitochondrial dysfunction. This review discusses the role of cholesterol, lysosomal and mitochondrial dysfunction, and ROS in the development and progression of hypercholesterolemic pregnancies.
Keywords: cholesterol traffic; lysosome; mitochondria; oxidative stress; placenta.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

