Superoxide Radicals in the Execution of Cell Death
- PMID: 35326151
- PMCID: PMC8944419
- DOI: 10.3390/antiox11030501
Superoxide Radicals in the Execution of Cell Death
Abstract
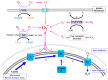
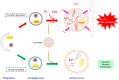
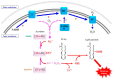
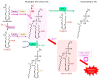
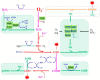
Superoxide is a primary oxygen radical that is produced when an oxygen molecule receives one electron. Superoxide dismutase (SOD) plays a primary role in the cellular defense against an oxidative insult by ROS. However, the resulting hydrogen peroxide is still reactive and, in the presence of free ferrous iron, may produce hydroxyl radicals and exacerbate diseases. Polyunsaturated fatty acids are the preferred target of hydroxyl radicals. Ferroptosis, a type of necrotic cell death induced by lipid peroxides in the presence of free iron, has attracted considerable interest because of its role in the pathogenesis of many diseases. Radical electrons, namely those released from mitochondrial electron transfer complexes, and those produced by enzymatic reactions, such as lipoxygenases, appear to cause lipid peroxidation. While GPX4 is the most potent anti-ferroptotic enzyme that is known to reduce lipid peroxides to alcohols, other antioxidative enzymes are also indirectly involved in protection against ferroptosis. Moreover, several low molecular weight compounds that include α-tocopherol, ascorbate, and nitric oxide also efficiently neutralize radical electrons, thereby suppressing ferroptosis. The removal of radical electrons in the early stages is of primary importance in protecting against ferroptosis and other diseases that are related to oxidative stress.
Keywords: ferroptosis; nitric oxide; radical electron; superoxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Janssen-Heininger Y.M., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T., Stamler J.S., Rhee S.G., van der Vliet A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

