Kojic Acid Showed Consistent Inhibitory Activity on Tyrosinase from Mushroom and in Cultured B16F10 Cells Compared with Arbutins
- PMID: 35326152
- PMCID: PMC8944748
- DOI: 10.3390/antiox11030502
Kojic Acid Showed Consistent Inhibitory Activity on Tyrosinase from Mushroom and in Cultured B16F10 Cells Compared with Arbutins
Abstract
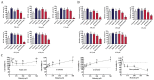
Kojic acid, β-arbutin, α-arbutin, and deoxyarbutin have been reported as tyrosinase inhibitors in many articles, but some contradictions exist in their differing results. In order to provide some explanations for these contradictions and to find the most suitable compound as a positive control for screening potential tyrosinase inhibitors, the activity and inhibition type of the aforementioned compounds on monophenolase and diphenolase of mushroom tyrosinase (MTYR) were studied. Their effects on B16F10 cells melanin content, tyrosinase (BTYR) activity, and cell viability were also exposed. Results indicated that α-arbutin competitively inhibited monophenolase activity, whereas they uncompetitively activated diphenolase activity of MTYR. β-arbutin noncompetitively and competitively inhibited monophenolase activity at high molarity (4000 µM) and moderate molarity (250-1000 µM) respectively, whereas it activated the diphenolase activity of MTYR. Deoxyarbutin competitively inhibited diphenolase activity, but could not inhibit monophenolase activity and only extended the lag time. Kojic acid competitively inhibited monophenolase activity and competitive-noncompetitive mixed-type inhibited diphenolase activity of MTYR. In a cellular experiment, deoxyarbutin effectively inhibited BTYR activity and reduced melanin content, but it also potently decreased cell viability. α-arbutin and β-arbutin dose-dependently inhibited BTYR activity, reduced melanin content, and increased cell viability. Kojic acid did not affect cell viability at 43.8-700 µM, but inhibited BTYR activity and reduced melanin content in a dose-dependent manner. Therefore, kojic acid was considered as the most suitable positive control among these four compounds, because it could inhibit both monophenolase and diphenolase activity of MTYR and reduce intercellular melanin content by inhibiting BTYR activity without cytotoxicity. Some explanations for the contradictions in the reported articles were provided.
Keywords: cell viability; deoxyarbutin; diphenolase activity; inhibition type; kojic acid; melanin content; monophenolase activity; tyrosinases; α-arbutin; β-arbutin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dual effects of alpha-arbutin on monophenolase and diphenolase activities of mushroom tyrosinase.PLoS One. 2014 Oct 10;9(10):e109398. doi: 10.1371/journal.pone.0109398. eCollection 2014. PLoS One. 2014. PMID: 25303458 Free PMC article.
-
Inhibitory Activities of Samples on Tyrosinases Were Affected by Enzyme Species and Sample Addition Methods.Int J Mol Sci. 2023 Mar 23;24(7):6013. doi: 10.3390/ijms24076013. Int J Mol Sci. 2023. PMID: 37046986 Free PMC article.
-
Characterization of inhibitory effects of the potential therapeutic inhibitors, benzoic acid and pyridine derivatives, on the monophenolase and diphenolase activities of tyrosinase.Iran J Basic Med Sci. 2015 Feb;18(2):122-9. Iran J Basic Med Sci. 2015. PMID: 25810885 Free PMC article.
-
Novel synthetic kojic acid-methimazole derivatives inhibit mushroom tyrosinase and melanogenesis.J Biosci Bioeng. 2016 Dec;122(6):666-672. doi: 10.1016/j.jbiosc.2016.06.002. Epub 2016 Jun 25. J Biosci Bioeng. 2016. PMID: 27353860
-
Biotechnological production of arbutins (α- and β-arbutins), skin-lightening agents, and their derivatives.Appl Microbiol Biotechnol. 2012 Sep;95(6):1417-25. doi: 10.1007/s00253-012-4297-4. Epub 2012 Jul 29. Appl Microbiol Biotechnol. 2012. PMID: 22843425 Review.
Cited by
-
Inhibitory Effects of Polyphenol- and Flavonoid-Enriched Rice Seed Extract on Melanogenesis in Melan-a Cells via MAPK Signaling-Mediated MITF Downregulation.Int J Mol Sci. 2023 Jul 24;24(14):11841. doi: 10.3390/ijms241411841. Int J Mol Sci. 2023. PMID: 37511600 Free PMC article.
-
In Silico Identification and Molecular Mechanism of Novel Tyrosinase Inhibitory Peptides Derived from Nacre of Pinctada martensii.Mar Drugs. 2024 Aug 7;22(8):359. doi: 10.3390/md22080359. Mar Drugs. 2024. PMID: 39195475 Free PMC article.
-
Effects of pine needle essential oil on melanin synthesis in B16F10 cells and its mechanism.Biomed Rep. 2025 Jun 6;23(2):133. doi: 10.3892/br.2025.2011. eCollection 2025 Aug. Biomed Rep. 2025. PMID: 40530398 Free PMC article.
-
The in cellular and in vivo melanogenesis inhibitory activity of safflospermidines from Helianthus annuus L. bee pollen in B16F10 murine melanoma cells and zebrafish embryos.PLoS One. 2025 Jun 24;20(6):e0325264. doi: 10.1371/journal.pone.0325264. eCollection 2025. PLoS One. 2025. PMID: 40554513 Free PMC article.
-
Computer-Aided Virtual Screening and In Vitro Validation of Biomimetic Tyrosinase Inhibitory Peptides from Abalone Peptidome.Int J Mol Sci. 2023 Feb 5;24(4):3154. doi: 10.3390/ijms24043154. Int J Mol Sci. 2023. PMID: 36834568 Free PMC article.
References
-
- Wang W., Chen L., Wang W.W., Zhang J., Jiang H. Inhibition of active compounds in tea on melanin formation. J. Tea Sci. 2021;41:7–18. doi: 10.13305/j.cnki.jts.20201209.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

