Apoptosis-Inducing Factor Deficiency Induces Tissue-Specific Alterations in Autophagy: Insights from a Preclinical Model of Mitochondrial Disease and Exercise Training Effects
- PMID: 35326160
- PMCID: PMC8944439
- DOI: 10.3390/antiox11030510
Apoptosis-Inducing Factor Deficiency Induces Tissue-Specific Alterations in Autophagy: Insights from a Preclinical Model of Mitochondrial Disease and Exercise Training Effects
Abstract
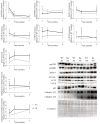
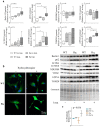
We analyzed the effects of apoptosis-inducing factor (AIF) deficiency, as well as those of an exercise training intervention on autophagy across tissues (heart, skeletal muscle, cerebellum and brain), that are primarily affected by mitochondrial diseases, using a preclinical model of these conditions, the Harlequin (Hq) mouse. Autophagy markers were analyzed in: (i) 2, 3 and 6 month-old male wild-type (WT) and Hq mice, and (ii) WT and Hq male mice that were allocated to an exercise training or sedentary group. The exercise training started upon onset of the first symptoms of ataxia in Hq mice and lasted for 8 weeks. Higher content of autophagy markers and free amino acids, and lower levels of sarcomeric proteins were found in the skeletal muscle and heart of Hq mice, suggesting increased protein catabolism. Leupeptin-treatment demonstrated normal autophagic flux in the Hq heart and the absence of mitophagy. In the cerebellum and brain, a lower abundance of Beclin 1 and ATG16L was detected, whereas higher levels of the autophagy substrate p62 and LAMP1 levels were observed in the cerebellum. The exercise intervention did not counteract the autophagy alterations found in any of the analyzed tissues. In conclusion, AIF deficiency induces tissue-specific alteration of autophagy in the Hq mouse, with accumulation of autophagy markers and free amino acids in the heart and skeletal muscle, but lower levels of autophagy-related proteins in the cerebellum and brain. Exercise intervention, at least if starting when muscle atrophy and neurological symptoms are already present, is not sufficient to mitigate autophagy perturbations.
Keywords: Harlequin; OXPHOS; autophagy; brain; cerebellum; heart; mitochondrial diseases; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Exercise Training and Neurodegeneration in Mitochondrial Disorders: Insights From the Harlequin Mouse.Front Physiol. 2020 Dec 8;11:594223. doi: 10.3389/fphys.2020.594223. eCollection 2020. Front Physiol. 2020. PMID: 33363476 Free PMC article.
-
Physical Exercise and Mitochondrial Disease: Insights From a Mouse Model.Front Neurol. 2019 Jul 25;10:790. doi: 10.3389/fneur.2019.00790. eCollection 2019. Front Neurol. 2019. PMID: 31402893 Free PMC article.
-
Pathophysiology of Cerebellar Degeneration in Mitochondrial Disorders: Insights from the Harlequin Mouse.Int J Mol Sci. 2023 Jun 30;24(13):10973. doi: 10.3390/ijms241310973. Int J Mol Sci. 2023. PMID: 37446148 Free PMC article.
-
Mutation frequency is not elevated in the cerebellum of harlequin/Big Blue(®) mice but Class II deletions occur preferentially in young harlequin cerebellum.Mutat Res. 2011 Feb 10;707(1-2):53-60. doi: 10.1016/j.mrfmmm.2010.12.012. Epub 2010 Dec 30. Mutat Res. 2011. PMID: 21195094
-
A deficiency of apoptosis inducing factor (AIF) in Harlequin mouse heart mitochondria paradoxically reduces ROS generation during ischemia-reperfusion.Front Physiol. 2014 Jul 22;5:271. doi: 10.3389/fphys.2014.00271. eCollection 2014. Front Physiol. 2014. PMID: 25101006 Free PMC article.
References
-
- Emperador S., Garrido-Pérez N., Amezcua-Gil J., Gaudó P., Andrés-Sanz J.A., Yubero D., Fernández-Marmiesse A., O’Callaghan M.M., Escobar J.D.O., Iriondo M., et al. Molecular Characterization of New FBXL4 Mutations in Patients with mtDNA Depletion Syndrome. Front. Genet. 2019;10:1300. doi: 10.3389/fgene.2019.01300. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

