Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats
- PMID: 35326164
- PMCID: PMC8944720
- DOI: 10.3390/antiox11030514
Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats
Abstract
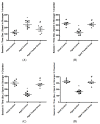
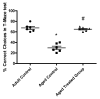
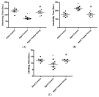
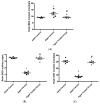
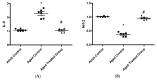
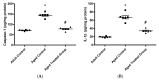
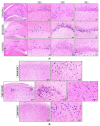
Aging is a naturally occurring process inevitably affecting each living human. The brain is adversely affected by aging with increased risks of developing various neurological disorders. Thus, it is essential to investigate practical approaches that can counteract the impact of aging on the brain. Vitamin K2 (Vit. K2) is a naturally occurring vitamin with reported valuable therapeutic effects. The current study highlights the role of Vit. K2 administration in counteracting age-related changes in the brain using naturally aging rats. Three-month-old rats were assigned to two groups: an ageing control group receiving a drug vehicle and an ageing group orally gavaged with Vit. K2 (30 mg/kg, once daily 5 days per week). Treatment was continued for 17 months. Ten three-month-old rats were used as the adult control. Vit. K2 improved functional performance, reduced social anxiety, depressive-like behavior, and enhanced memory performance with concomitant preservation of hippocampal and cerebral cortex tyrosine hydroxylase expression. Biochemically, Vit. K2 administration restored oxidative-anti-oxidative homeostasis in the brain. Vit. K2 modulated inflammatory signaling, as evidenced by suppression in the brain of NLRP3, caspase-1, Il-1β, TNFα, IL-6, and CD68 expression. Concomitantly, histopathological examination revealed consistent hippocampal and cerebral cortex improvement. Thus, it can be inferred that Vit K2 can slow down age-related changes in the brain associated with modulation of NLRP3/caspase-1/Nrf-2 signaling.
Keywords: NLRP3; Vit. K2; aging; cerebral; hippocampus; tyrosine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

