Haloperoxidase-Catalyzed Luminol Luminescence
- PMID: 35326168
- PMCID: PMC8944799
- DOI: 10.3390/antiox11030518
Haloperoxidase-Catalyzed Luminol Luminescence
Abstract
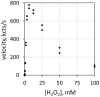
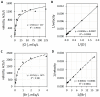
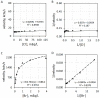
Common peroxidase action and haloperoxidase action are quantifiable as light emission from dioxygenation of luminol (5-amino-2,3-dihydrophthalazine-1,4-dione). The velocity of enzyme action is dependent on the concentration of reactants. Thus, the reaction order of each participant reactant in luminol luminescence was determined. Horseradish peroxidase (HRP)-catalyzed luminol luminescence is first order for hydrogen peroxide (H2O2), but myeloperoxidase (MPO) and eosinophil peroxidase (EPO) are second order for H2O2. For MPO, reaction is first order for chloride (Cl-) or bromide (Br-). For EPO, reaction is first order for Br-. HRP action has no halide requirement. For MPO and EPO, reaction is first order for luminol, but for HRP, reaction is greater than first order for luminol. Haloperoxidase-catalyzed luminol luminescence requires acidity, but HRP action requires alkalinity. Unlike the radical mechanism of common peroxidase, haloperoxidases (XPO) catalyze non-radical oxidation of halide to hypohalite. That reaction is second order for H2O2 is consistent with the non-enzymatic reaction of hypohalite with a second H2O2 to produce singlet molecular oxygen (1O2*) for luminol dioxygenation. Alternatively, luminol dehydrogenation by hypohalite followed by reaction with H2O2 yields dioxygenation consistent with the same reaction order. Haloperoxidase action, Cl-, and Br- are specifically quantifiable as luminol luminescence in an acidic milieu.
Keywords: chemiluminescence; eosinophil peroxidase; halide oxidation; haloperoxidase; horseradish peroxidase; kinetic analysis; luminol luminescence; myeloperoxidase; reaction order; singlet molecular oxygen.
Conflict of interest statement
Exoxemis, Inc produces MPO and EPO and owns intellectual property (IP) regarding the diagnostic and therapeutic applications of haloperoxidases. I am the inventor of IP described in patent US005556758A that was filed in 1994 and issued in 1996. I continue to consult for Exoxemis, Inc but I do not own this IP.
Figures



References
-
- Kasha M., Khan A.U. The physics, chemistry, and biology of singlet molecular oxygen. Ann. N. Y. Acad. Sci. 1970;171:5–23. doi: 10.1111/j.1749-6632.1970.tb39294.x. - DOI
-
- Held A., Halko D., Hurst J. Mechanisms of chlorine oxidation of hydrogen peroxide. J. Am. Chem. Soc. 1978;100:5732–5740. doi: 10.1021/ja00486a025. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

