Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders
- PMID: 35326205
- PMCID: PMC8944973
- DOI: 10.3390/antiox11030555
Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders
Abstract
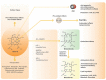
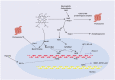
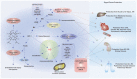
The heme oxygenase (HO) enzyme system catabolizes heme to carbon monoxide (CO), ferrous iron, and biliverdin-IXα (BV), which is reduced to bilirubin-IXα (BR) by biliverdin reductase (BVR). HO activity is represented by two distinct isozymes, the inducible form, HO-1, and a constitutive form, HO-2, encoded by distinct genes (HMOX1, HMOX2, respectively). HO-1 responds to transcriptional activation in response to a wide variety of chemical and physical stimuli, including its natural substrate heme, oxidants, and phytochemical antioxidants. The expression of HO-1 is regulated by NF-E2-related factor-2 and counter-regulated by Bach-1, in a heme-sensitive manner. Additionally, HMOX1 promoter polymorphisms have been associated with human disease. The induction of HO-1 can confer protection in inflammatory conditions through removal of heme, a pro-oxidant and potential catalyst of lipid peroxidation, whereas iron released from HO activity may trigger ferritin synthesis or ferroptosis. The production of heme-derived reaction products (i.e., BV, BR) may contribute to HO-dependent cytoprotection via antioxidant and immunomodulatory effects. Additionally, BVR and BR have newly recognized roles in lipid regulation. CO may alter mitochondrial function leading to modulation of downstream signaling pathways that culminate in anti-apoptotic, anti-inflammatory, anti-proliferative and immunomodulatory effects. This review will present evidence for beneficial effects of HO-1 and its reaction products in human diseases, including cardiovascular disease (CVD), metabolic conditions, including diabetes and obesity, as well as acute and chronic diseases of the liver, kidney, or lung. Strategies targeting the HO-1 pathway, including genetic or chemical modulation of HO-1 expression, or application of BR, CO gas, or CO donor compounds show therapeutic potential in inflammatory conditions, including organ ischemia/reperfusion injury. Evidence from human studies indicate that HO-1 expression may represent a biomarker of oxidative stress in various clinical conditions, while increases in serum BR levels have been correlated inversely to risk of CVD and metabolic disease. Ongoing human clinical trials investigate the potential of CO as a therapeutic in human disease.
Keywords: bilirubin; carbon monoxide; cardiovascular disease; heme oxygenase; inflammation; kidney disease; lung disease; metabolic disease.
Conflict of interest statement
Stefan Ryter holds interests in Proterris Inc., a company involved in commercial applications of iCO. Currently, Ryter is a senior scientist for Proterris, Inc. (Boston), a company that develops iCO for human therapeutic applications. Ryter is also an affiliate of Weill Cornell Medicine, and a consultant of Baylor College of Medicine. The company had no role in the writing of the manuscript, or in the decision to publish the result.
Figures
Similar articles
-
Therapeutic Potential of Heme Oxygenase-1 and Carbon Monoxide in Acute Organ Injury, Critical Illness, and Inflammatory Disorders.Antioxidants (Basel). 2020 Nov 19;9(11):1153. doi: 10.3390/antiox9111153. Antioxidants (Basel). 2020. PMID: 33228260 Free PMC article. Review.
-
Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation.Transl Res. 2016 Jan;167(1):7-34. doi: 10.1016/j.trsl.2015.06.011. Epub 2015 Jun 23. Transl Res. 2016. PMID: 26166253 Free PMC article. Review.
-
Significance of Heme and Heme Degradation in the Pathogenesis of Acute Lung and Inflammatory Disorders.Int J Mol Sci. 2021 May 24;22(11):5509. doi: 10.3390/ijms22115509. Int J Mol Sci. 2021. PMID: 34073678 Free PMC article. Review.
-
Heme oxygenase-1: its therapeutic roles in inflammatory diseases.Immune Netw. 2009 Feb;9(1):12-9. doi: 10.4110/in.2009.9.1.12. Epub 2009 Feb 28. Immune Netw. 2009. PMID: 20107533 Free PMC article.
-
Heme Oxygenase-1 and Its Metabolites Carbon Monoxide and Biliverdin, but Not Iron, Exert Antiviral Activity against Porcine Circovirus Type 3.Microbiol Spectr. 2023 Jun 15;11(3):e0506022. doi: 10.1128/spectrum.05060-22. Epub 2023 May 4. Microbiol Spectr. 2023. PMID: 37140466 Free PMC article.
Cited by
-
Preventive Electroacupuncture Alleviates Oxidative Stress and Inflammation via Keap1/Nrf2/HO-1 Pathway in Rats with Cyclophosphamide-Induced Premature Ovarian Insufficiency.Biomed Res Int. 2022 Aug 25;2022:6718592. doi: 10.1155/2022/6718592. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2023 Aug 2;2023:9816950. doi: 10.1155/2023/9816950. PMID: 36060148 Free PMC article. Retracted.
-
A Descriptive Review of the Antioxidant Effects and Mechanisms of Action of Berberine and Silymarin.Molecules. 2024 Sep 26;29(19):4576. doi: 10.3390/molecules29194576. Molecules. 2024. PMID: 39407506 Free PMC article. Review.
-
Molecular Docking Identifies 1,8-Cineole (Eucalyptol) as A Novel PPARγ Agonist That Alleviates Colon Inflammation.Int J Mol Sci. 2023 Mar 24;24(7):6160. doi: 10.3390/ijms24076160. Int J Mol Sci. 2023. PMID: 37047133 Free PMC article.
-
Oroxylin A inhibits inflammatory cytokines in periodontitis via HO‑1.Mol Med Rep. 2024 Jul;30(1):126. doi: 10.3892/mmr.2024.13249. Epub 2024 May 24. Mol Med Rep. 2024. PMID: 38785151 Free PMC article.
-
Absence of Heme Oxygenase-1 Affects Trophoblastic Spheroid Implantation and Provokes Dysregulation of Stress and Angiogenesis Gene Expression in the Uterus.Cells. 2024 Feb 22;13(5):376. doi: 10.3390/cells13050376. Cells. 2024. PMID: 38474340 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

