Understanding Myeloperoxidase-Induced Damage to HDL Structure and Function in the Vessel Wall: Implications for HDL-Based Therapies
- PMID: 35326206
- PMCID: PMC8944857
- DOI: 10.3390/antiox11030556
Understanding Myeloperoxidase-Induced Damage to HDL Structure and Function in the Vessel Wall: Implications for HDL-Based Therapies
Abstract
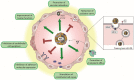
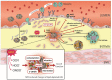
Atherosclerosis is a disease of increased oxidative stress characterized by protein and lipid modifications in the vessel wall. One important oxidative pathway involves reactive intermediates generated by myeloperoxidase (MPO), an enzyme present mainly in neutrophils and monocytes. Tandem MS analysis identified MPO as a component of lesion derived high-density lipoprotein (HDL), showing that the two interact in the arterial wall. MPO modifies apolipoprotein A1 (apoA-I), paraoxonase 1 and certain HDL-associated phospholipids in human atheroma. HDL isolated from atherosclerotic plaques depicts extensive MPO mediated posttranslational modifications, including oxidation of tryptophan, tyrosine and methionine residues, and carbamylation of lysine residues. In addition, HDL associated plasmalogens are targeted by MPO, generating 2-chlorohexadecanal, a pro-inflammatory and endothelial barrier disrupting lipid that suppresses endothelial nitric oxide formation. Lesion derived HDL is predominantly lipid-depleted and cross-linked and exhibits a nearly 90% reduction in lecithin-cholesterol acyltransferase activity and cholesterol efflux capacity. Here we provide a current update of the pathophysiological consequences of MPO-induced changes in the structure and function of HDL and discuss possible therapeutic implications and options. Preclinical studies with a fully functional apoA-I variant with pronounced resistance to oxidative inactivation by MPO-generated oxidants are currently ongoing. Understanding the relationships between pathophysiological processes that affect the molecular composition and function of HDL and associated diseases is central to the future use of HDL in diagnostics, therapy, and ultimately disease management.
Keywords: HDL; cholesterol efflux capacity; myeloperoxidase; paraoxonase; post-translational modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Myeloperoxidase Targets Apolipoprotein A-I for Site-Specific Tyrosine Chlorination in Atherosclerotic Lesions and Generates Dysfunctional High-Density Lipoprotein.Chem Res Toxicol. 2021 Jun 21;34(6):1672-1680. doi: 10.1021/acs.chemrestox.1c00086. Epub 2021 Apr 16. Chem Res Toxicol. 2021. PMID: 33861588
-
The pattern of apolipoprotein A-I lysine carbamylation reflects its lipidation state and the chemical environment within human atherosclerotic aorta.J Biol Chem. 2022 Apr;298(4):101832. doi: 10.1016/j.jbc.2022.101832. Epub 2022 Mar 15. J Biol Chem. 2022. PMID: 35304099 Free PMC article.
-
Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL.Biochim Biophys Acta. 2012 Mar;1821(3):490-501. doi: 10.1016/j.bbalip.2011.11.011. Epub 2011 Dec 10. Biochim Biophys Acta. 2012. PMID: 22178192 Free PMC article. Review.
-
Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein.Chem Res Toxicol. 2010 Mar 15;23(3):447-54. doi: 10.1021/tx9003775. Chem Res Toxicol. 2010. PMID: 20043647 Free PMC article. Review.
-
Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle.J Biol Chem. 2009 Nov 6;284(45):30825-35. doi: 10.1074/jbc.M109.047605. Epub 2009 Sep 2. J Biol Chem. 2009. PMID: 19726691 Free PMC article.
Cited by
-
Exploring Neutrophil Extracellular Traps in Cardiovascular Pathologies: The Impact of Lipid Profiles, PAD4, and Radiation.Biocell. 2025;49(6):931-959. doi: 10.32604/biocell.2025.062789. Epub 2025 Jun 24. Biocell. 2025. PMID: 40786884 Free PMC article.
-
Effects of Seven Weeks of Combined Physical Training on High-Density Lipoprotein Functionality in Overweight/Obese Subjects.Metabolites. 2023 Oct 10;13(10):1068. doi: 10.3390/metabo13101068. Metabolites. 2023. PMID: 37887393 Free PMC article.
-
Naturally Occurring Organohalogen Compounds-A Comprehensive Review.Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review.
-
HDL Function and Atherosclerosis: Reactive Dicarbonyls as Promising Targets of Therapy.Circ Res. 2023 May 26;132(11):1521-1545. doi: 10.1161/CIRCRESAHA.123.321563. Epub 2023 May 25. Circ Res. 2023. PMID: 37228232 Free PMC article. Review.
-
The Alteration of HDL in Patients with AMI Inhibited Angiogenesis by Blocking ERK1/2 Activation.Cardiovasc Ther. 2022 Aug 16;2022:1057772. doi: 10.1155/2022/1057772. eCollection 2022. Cardiovasc Ther. 2022. PMID: 36072560 Free PMC article.
References
-
- Segal A.W. Physiology and Pathology of the Neutrophil. Nature. 1979;278:195. doi: 10.1038/278195b0. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

