A Metabolic Network Mediating the Cycling of Succinate, a Product of ROS Detoxification into α-Ketoglutarate, an Antioxidant
- PMID: 35326210
- PMCID: PMC8945261
- DOI: 10.3390/antiox11030560
A Metabolic Network Mediating the Cycling of Succinate, a Product of ROS Detoxification into α-Ketoglutarate, an Antioxidant
Abstract
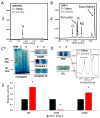
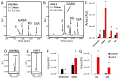
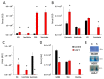
Sulfur is an essential element for life. However, the soil microbe Pseudomonas (P.) fluorescens can survive in a low sulfur environment. When cultured in a sulfur-deficient medium, the bacterium reprograms its metabolic pathways to produce α-ketoglutarate (KG) and regenerate this keto-acid from succinate, a by-product of ROS detoxification. Succinate semialdehyde dehydrogenase (SSADH) and KG decarboxylase (KGDC) work in partnership to synthesize KG. This process is further aided by the increased activity of the enzymes glutamate decarboxylase (GDC) and γ-amino-butyrate transaminase (GABAT). The pool of succinate semialdehyde (SSA) generated is further channeled towards the formation of the antioxidant. Spectrophotometric analyses, HPLC experiments and electrophoretic studies with intact cells and cell-free extracts (CFE) pointed to the metabolites (succinate, SSA, GABA) and enzymes (SSADH, GDC, KGDC) contributing to this KG-forming metabolic machinery. Real-time polymerase chain reaction (RT-qPCR) revealed significant increase in transcripts of such enzymes as SSADH, GDC and KGDC. The findings of this study highlight a novel pathway involving keto-acids in ROS scavenging. The cycling of succinate into KG provides an efficient means of combatting an oxidative environment. Considering the central role of KG in biological processes, this metabolic network may be operative in other living systems.
Keywords: KG; glutamate decarboxylase; metabolic network; oxidative stress; succinate recycling; sulfur stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

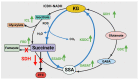
 = Cycle producing KG from glutamate,
= Cycle producing KG from glutamate,  = Regeneration of KG from succinate, the product of ROS detoxification.
= Regeneration of KG from succinate, the product of ROS detoxification.Similar articles
-
Isocitrate Lyase and Succinate Semialdehyde Dehydrogenase Mediate the Synthesis of α-Ketoglutarate in Pseudomonas fluorescens.Front Microbiol. 2019 Aug 23;10:1929. doi: 10.3389/fmicb.2019.01929. eCollection 2019. Front Microbiol. 2019. PMID: 31507554 Free PMC article.
-
Neuroinformatics analyses reveal GABAt and SSADH as major proteins involved in anticonvulsant activity of valproic acid.Biomed Pharmacother. 2016 Jul;81:402-410. doi: 10.1016/j.biopha.2016.04.036. Epub 2016 Apr 27. Biomed Pharmacother. 2016. PMID: 27261619
-
Mutants of GABA transaminase (POP2) suppress the severe phenotype of succinic semialdehyde dehydrogenase (ssadh) mutants in Arabidopsis.PLoS One. 2008;3(10):e3383. doi: 10.1371/journal.pone.0003383. Epub 2008 Oct 10. PLoS One. 2008. PMID: 18846220 Free PMC article.
-
Biochemical pathways to α-ketoglutarate, a multi-faceted metabolite.World J Microbiol Biotechnol. 2020 Jul 20;36(8):123. doi: 10.1007/s11274-020-02900-8. World J Microbiol Biotechnol. 2020. PMID: 32686016 Review.
-
Murine succinate semialdehyde dehydrogenase deficiency.Ann Neurol. 2003;54 Suppl 6:S81-90. doi: 10.1002/ana.10625. Ann Neurol. 2003. PMID: 12891658 Review.
Cited by
-
Mechanism and application of yeast and its culture in regulating intestinal antioxidant defense in ruminants.Front Vet Sci. 2025 Aug 7;12:1657244. doi: 10.3389/fvets.2025.1657244. eCollection 2025. Front Vet Sci. 2025. PMID: 40852435 Free PMC article. Review.
References
-
- Middaugh J., Hamel R., Jean-Baptiste G., Beriault R., Chenier D., Appanna V.D. Aluminum triggers decreased aconitase activity via Fe-S cluster disruption and the overexpression of isocitrate dehydrogenase and isocitrate lyase: A metabolic network mediating cellular survival. J. Biol. Chem. 2005;280:3159–3165. doi: 10.1074/jbc.M411979200. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

