Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Beyond Lipids: The Role in Oxidative Stress and Thrombosis
- PMID: 35326219
- PMCID: PMC8945358
- DOI: 10.3390/antiox11030569
Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Beyond Lipids: The Role in Oxidative Stress and Thrombosis
Abstract
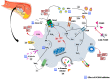
Proprotein convertase subtilisin/kexin type 9 (PCSK9), mainly secreted in the liver, is a key regulator of cholesterol homeostasis inducing LDL receptors' degradation. Beyond lipid metabolism, PCSK9 is involved in the development of atherosclerosis, promoting plaque formation in mice and human, impairing the integrity of endothelial monolayer and promoting the events that induce atherosclerosis disease progression. In addition, the PCSK9 ancillary role in the atherothrombosis process is widely debated. Indeed, recent evidence showed a regulatory effect of PCSK9 on redox system and platelet activation. In particular, the role of PCSK9 in the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox2) system, of MAP-kinase cascades and of CD36 and LOX-1 downstream pathways, suggests that PCSK9 may be a significant cofactor in atherothrombosis development. This evidence suggests that the serum levels of PCSK9 could represent a new biomarker for the occurrence of cardiovascular events. Finally, other evidence showed that PCSK9 inhibitors, a novel pharmacological tool introduced in clinical practice in recent years, counteracted these phenomena. In this review, we summarize the evidence concerning the role of PCSK9 in promoting oxidative-stress-related atherothrombotic process.
Keywords: anti-PCSK9; oxidative stress; platelets; proprotein convertase subtilisin/kexin type 9 (PCSK9); thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
PCSK9 Biology and Its Role in Atherothrombosis.Int J Mol Sci. 2021 May 30;22(11):5880. doi: 10.3390/ijms22115880. Int J Mol Sci. 2021. PMID: 34070931 Free PMC article. Review.
-
Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review.
-
PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36.Circulation. 2021 Jan 5;143(1):45-61. doi: 10.1161/CIRCULATIONAHA.120.046290. Epub 2020 Sep 29. Circulation. 2021. PMID: 32988222
-
Cyclase-associated protein 1 is a binding partner of proprotein convertase subtilisin/kexin type-9 and is required for the degradation of low-density lipoprotein receptors by proprotein convertase subtilisin/kexin type-9.Eur Heart J. 2020 Jan 7;41(2):239-252. doi: 10.1093/eurheartj/ehz566. Eur Heart J. 2020. PMID: 31419281 Free PMC article.
-
Emerging Insights on the Diverse Roles of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Chronic Liver Diseases: Cholesterol Metabolism and Beyond.Int J Mol Sci. 2022 Jan 19;23(3):1070. doi: 10.3390/ijms23031070. Int J Mol Sci. 2022. PMID: 35162992 Free PMC article. Review.
Cited by
-
The Pleiotropic Effects of Lipid-Modifying Interventions: Exploring Traditional and Emerging Hypolipidemic Therapies.Metabolites. 2024 Jul 17;14(7):388. doi: 10.3390/metabo14070388. Metabolites. 2024. PMID: 39057711 Free PMC article. Review.
-
Investigation of the interplay of PCSK9, cardiac dynamics, oxidative stress in coronary artery disease: case-control study.Front Endocrinol (Lausanne). 2025 Apr 28;16:1494438. doi: 10.3389/fendo.2025.1494438. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40357205 Free PMC article.
-
Prakriti elucidates the inter-individual variability in coronary artery disease risk-predicting biomarkers: A tertiary care hospital-based case control study.J Ayurveda Integr Med. 2025 Aug 18;16(5):101178. doi: 10.1016/j.jaim.2025.101178. Online ahead of print. J Ayurveda Integr Med. 2025. PMID: 40829435 Free PMC article.
-
Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease.Antioxidants (Basel). 2024 Apr 12;13(4):455. doi: 10.3390/antiox13040455. Antioxidants (Basel). 2024. PMID: 38671903 Free PMC article. Review.
-
Human CD36: Gene Regulation, Protein Function, and Its Role in Atherosclerosis Pathogenesis.Genes (Basel). 2025 Jun 13;16(6):705. doi: 10.3390/genes16060705. Genes (Basel). 2025. PMID: 40565598 Free PMC article. Review.
References
-
- Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E., Hegele R.A., Krauss R.M., Raal F.J., Schunkert H., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. - DOI - PMC - PubMed
-
- Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., Daemen M.J., Demer L.L., Hegele R.A., Nicholls S.J., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

