The Antioxidant Selenoprotein T Mimetic, PSELT, Induces Preconditioning-like Myocardial Protection by Relieving Endoplasmic-Reticulum Stress
- PMID: 35326221
- PMCID: PMC8944960
- DOI: 10.3390/antiox11030571
The Antioxidant Selenoprotein T Mimetic, PSELT, Induces Preconditioning-like Myocardial Protection by Relieving Endoplasmic-Reticulum Stress
Abstract
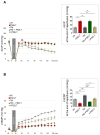
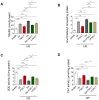
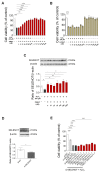
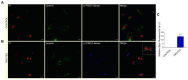
Oxidative stress and endoplasmic reticulum stress (ERS) are strictly involved in myocardial ischemia/reperfusion (MI/R). Selenoprotein T (SELENOT), a vital thioredoxin-like selenoprotein, is crucial for ER homeostasis and cardiomyocyte differentiation and protection, likely acting as a redox-sensing protein during MI/R. Here, we designed a small peptide (PSELT), encompassing the redox site of SELENOT, and investigated whether its pre-conditioning cardioprotective effect resulted from modulating ERS during I/R. The Langendorff rat heart model was employed for hemodynamic analysis, while mechanistic studies were performed in perfused hearts and H9c2 cardiomyoblasts. PSELT improved the post-ischemic contractile recovery, reducing infarct size and LDH release with and without the ERS inducer tunicamycin (TM). Mechanistically, I/R and TM upregulated SELENOT expression, which was further enhanced by PSELT. PSELT also prevented the expression of the ERS markers CHOP and ATF6, reduced cardiac lipid peroxidation and protein oxidation, and increased SOD and catalase activities. An inert PSELT (I-PSELT) lacking selenocysteine was ineffective. In H9c2 cells, H2O2 decreased cell viability and SELENOT expression, while PSELT rescued protein levels protecting against cell death. In SELENOT-deficient H9c2 cells, H2O2 exacerbated cell death, that was partially mitigated by PSELT. Microscopy analysis revealed that a fluorescent form of PSELT was internalized into cardiomyocytes with a perinuclear distribution. Conclusions: The cell-permeable PSELT is able to induce pharmacological preconditioning cardioprotection by mitigating ERS and oxidative stress, and by regulating endogenous SELENOT.
Keywords: antioxidants; heart; ischemia/reperfusion injury; peptides; selenoproteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The redox-active defensive Selenoprotein T as a novel stress sensor protein playing a key role in the pathophysiology of heart failure.J Transl Med. 2024 Apr 20;22(1):375. doi: 10.1186/s12967-024-05192-w. J Transl Med. 2024. PMID: 38643121 Free PMC article.
-
Palmitate-Induced Cardiac Lipotoxicity Is Relieved by the Redox-Active Motif of SELENOT through Improving Mitochondrial Function and Regulating Metabolic State.Cells. 2023 Mar 29;12(7):1042. doi: 10.3390/cells12071042. Cells. 2023. PMID: 37048116 Free PMC article.
-
A selenoprotein T-derived peptide protects the heart against ischaemia/reperfusion injury through inhibition of apoptosis and oxidative stress.Acta Physiol (Oxf). 2018 Aug;223(4):e13067. doi: 10.1111/apha.13067. Epub 2018 Apr 17. Acta Physiol (Oxf). 2018. PMID: 29575758
-
Progress in the emerging role of selenoproteins in cardiovascular disease: focus on endoplasmic reticulum-resident selenoproteins.Cell Mol Life Sci. 2019 Oct;76(20):3969-3985. doi: 10.1007/s00018-019-03195-1. Epub 2019 Jun 19. Cell Mol Life Sci. 2019. PMID: 31218451 Free PMC article. Review.
-
Selenoprotein T: An Essential Oxidoreductase Serving as a Guardian of Endoplasmic Reticulum Homeostasis.Antioxid Redox Signal. 2020 Dec 10;33(17):1257-1275. doi: 10.1089/ars.2019.7931. Epub 2020 Jul 7. Antioxid Redox Signal. 2020. PMID: 32524825 Review.
Cited by
-
The redox-active defensive Selenoprotein T as a novel stress sensor protein playing a key role in the pathophysiology of heart failure.J Transl Med. 2024 Apr 20;22(1):375. doi: 10.1186/s12967-024-05192-w. J Transl Med. 2024. PMID: 38643121 Free PMC article.
-
A recombinant fragment antigen-binding (Fab) of trastuzumab displays low cytotoxic profile in adult human cardiomyocytes: first evidence and the key implication of FcγRIIA receptor.Acta Pharmacol Sin. 2025 Mar;46(3):618-631. doi: 10.1038/s41401-024-01397-3. Epub 2024 Oct 16. Acta Pharmacol Sin. 2025. PMID: 39414958
-
Selenium and Skeletal Muscle Health in Sports Nutrition.Nutrients. 2025 May 31;17(11):1902. doi: 10.3390/nu17111902. Nutrients. 2025. PMID: 40507171 Free PMC article. Review.
-
Quercetin and Its Derivative Counteract Palmitate-Dependent Lipotoxicity by Inhibiting Oxidative Stress and Inflammation in Cardiomyocytes.Int J Environ Res Public Health. 2023 Feb 16;20(4):3492. doi: 10.3390/ijerph20043492. Int J Environ Res Public Health. 2023. PMID: 36834186 Free PMC article.
-
Specialized Pro-Resolving Mediators as Emerging Players in Cardioprotection: From Inflammation Resolution to Therapeutic Potential.Acta Physiol (Oxf). 2025 Jul;241(7):e70062. doi: 10.1111/apha.70062. Acta Physiol (Oxf). 2025. PMID: 40433738 Free PMC article. Review.
References
-
- Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. - DOI - PubMed
-
- Hausenloy D.J., Barrabes J.A., Bøtker H.E., Davidson S.M., Di Lisa F., Downey J., Engstrom T., Ferdinandy P., Carbrera-Fuentes H.A., Heusch G., et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016;111:70. doi: 10.1007/s00395-016-0588-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

