Squalene Loaded Nanoparticles Effectively Protect Hepatic AML12 Cell Lines against Oxidative and Endoplasmic Reticulum Stress in a TXNDC5-Dependent Way
- PMID: 35326231
- PMCID: PMC8945349
- DOI: 10.3390/antiox11030581
Squalene Loaded Nanoparticles Effectively Protect Hepatic AML12 Cell Lines against Oxidative and Endoplasmic Reticulum Stress in a TXNDC5-Dependent Way
Abstract
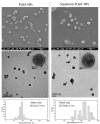
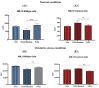
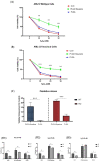
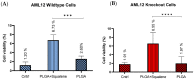
Virgin olive oil, the main source of fat in the Mediterranean diet, contains a substantial amount of squalene which possesses natural antioxidant properties. Due to its highly hydrophobic nature, its bioavailability is reduced. In order to increase its delivery and potentiate its actions, squalene has been loaded into PLGA nanoparticles (NPs). The characterization of the resulting nanoparticles was assessed by electron microscopy, dynamic light scattering, zeta potential and high-performance liquid chromatography. Reactive oxygen species (ROS) generation and cell viability assays were carried out in AML12 (alpha mouse liver cell line) and a TXNDC5-deficient AML12 cell line (KO), which was generated by CRISPR/cas9 technology. According to the results, squalene was successfully encapsulated in PLGA NPs, and had rapid and efficient cellular uptake at 30 µM squalene concentration. Squalene reduced ROS in AML12, whereas ROS levels increased in KO cells and improved cell viability in both when subjected to oxidative stress by significant induction of Gpx4. Squalene enhanced cell viability in ER-induced stress by decreasing Ern1 or Eif2ak3 expressions. In conclusion, TXNDC5 shows a crucial role in regulating ER-induced stress through different signaling pathways, and squalene protects mouse hepatocytes from oxidative and endoplasmic reticulum stresses by several molecular mechanisms depending on TXNDC5.
Keywords: Eif2ak3; Ern1; Gpx4; PLGA; TXNDC5; endoplasmic reticulum stress; liver; olive oil; oxidative stress; squalene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
TXNDC5 Plays a Crucial Role in Regulating Endoplasmic Reticulum Activity through Different ER Stress Signaling Pathways in Hepatic Cells.Int J Mol Sci. 2024 Jun 28;25(13):7128. doi: 10.3390/ijms25137128. Int J Mol Sci. 2024. PMID: 39000233 Free PMC article.
-
Thioredoxin Domain Containing 5 (TXNDC5): Friend or Foe?Curr Issues Mol Biol. 2024 Apr 4;46(4):3134-3163. doi: 10.3390/cimb46040197. Curr Issues Mol Biol. 2024. PMID: 38666927 Free PMC article. Review.
-
Chitosan Nanoparticles, a Novel Drug Delivery System to Transfer Squalene for Hepatocyte Stress Protection.ACS Omega. 2024 Dec 16;9(52):51379-51393. doi: 10.1021/acsomega.4c08258. eCollection 2024 Dec 31. ACS Omega. 2024. PMID: 39758614 Free PMC article.
-
Thioredoxin domain containing 5 is involved in the hepatic storage of squalene into lipid droplets in a sex-specific way.J Nutr Biochem. 2024 Feb;124:109503. doi: 10.1016/j.jnutbio.2023.109503. Epub 2023 Oct 26. J Nutr Biochem. 2024. PMID: 37898391
-
Decoding cell death signals in liver inflammation.J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
Cited by
-
Endoplasmic Reticulum Protein TXNDC5 Interacts with PRDX6 and HSPA9 to Regulate Glutathione Metabolism and Lipid Peroxidation in the Hepatic AML12 Cell Line.Int J Mol Sci. 2023 Dec 5;24(24):17131. doi: 10.3390/ijms242417131. Int J Mol Sci. 2023. PMID: 38138960 Free PMC article.
-
TXNDC5 Plays a Crucial Role in Regulating Endoplasmic Reticulum Activity through Different ER Stress Signaling Pathways in Hepatic Cells.Int J Mol Sci. 2024 Jun 28;25(13):7128. doi: 10.3390/ijms25137128. Int J Mol Sci. 2024. PMID: 39000233 Free PMC article.
-
Enhancing the bioavailability and activity of natural antioxidants with nanobubbles and nanoparticles.Redox Rep. 2024 Dec;29(1):2333619. doi: 10.1080/13510002.2024.2333619. Epub 2024 Apr 5. Redox Rep. 2024. PMID: 38577911 Free PMC article. Review.
-
Thioredoxin Domain Containing 5 (TXNDC5): Friend or Foe?Curr Issues Mol Biol. 2024 Apr 4;46(4):3134-3163. doi: 10.3390/cimb46040197. Curr Issues Mol Biol. 2024. PMID: 38666927 Free PMC article. Review.
-
Olive Oil Antioxidants.Antioxidants (Basel). 2022 May 19;11(5):996. doi: 10.3390/antiox11050996. Antioxidants (Basel). 2022. PMID: 35624859 Free PMC article.
References
-
- Guasch-Ferré M., Li Y., Willett W.C., Sun Q., Sampson L., Salas-Salvadó J., Martínez-González M.A., Stampfer M.J., Hu F.B. Consumption of olive oil and risk of total and cause-specific mortality among US adults. J. Am. Coll. Cardiol. 2022;79:101–112. doi: 10.1016/j.jacc.2021.10.041. - DOI - PMC - PubMed
-
- Martínez-Beamonte R., Sánchez-Marco J., Felices M.J., Barranquero C., Gascón S., Arnal C., Burillo J.C., Lasheras R., Busto R., Lasunción M.A. Dietary squalene modifies plasma lipoproteins and hepatic cholesterol metabolism in rabbits. Food Funct. 2021;12:8141–8153. doi: 10.1039/D0FO01836H. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

