Computational Models on Pathological Redox Signalling Driven by Pregnancy: A Review
- PMID: 35326235
- PMCID: PMC8945226
- DOI: 10.3390/antiox11030585
Computational Models on Pathological Redox Signalling Driven by Pregnancy: A Review
Abstract
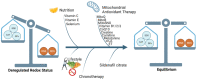
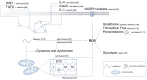
Oxidative stress is associated with a myriad of diseases including pregnancy pathologies with long-term cardiovascular repercussions for both the mother and baby. Aberrant redox signalling coupled with deficient antioxidant defence leads to chronic molecular impairment. Abnormal placentation has been considered the primary source for reactive species; however, placental dysfunction has been deemed secondary to maternal cardiovascular maladaptation in pregnancy. While various therapeutic interventions, aimed at combating deregulated oxidative stress during pregnancy have shown promise in experimental models, they often result as inconclusive or detrimental in clinical trials, warranting the need for further research to identify candidates. The strengths and limitations of current experimental methods in redox research are discussed. Assessment of redox status and oxidative stress in experimental models and in clinical practice remains challenging; the state-of-the-art of computational models in this field is presented in this review, comparing static and dynamic models which provide functional information such as protein-protein interactions, as well as the impact of changes in molecular species on the redox-status of the system, respectively. Enhanced knowledge of redox biology in during pregnancy through computational modelling such as generation of Systems Biology Markup Language model which integrates existing models to a larger network in the context of placenta physiology.
Keywords: antioxidant therapy; cardio-obstetrics; integrative modelling; oxidative stress; preeclampsia; systems biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

