Fragile X Mental Retardation Protein and Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome: A Pilot Study
- PMID: 35326270
- PMCID: PMC8946825
- DOI: 10.3390/brainsci12030314
Fragile X Mental Retardation Protein and Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome: A Pilot Study
Abstract
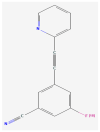
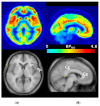
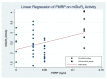
Multiple lines of evidence suggest that a deficiency of Fragile X Mental Retardation Protein (FMRP) mediates dysfunction of the metabotropic glutamate receptor subtype 5 (mGluR5) in the pathogenesis of fragile X syndrome (FXS), the most commonly known single-gene cause of inherited intellectual disability (ID) and autism spectrum disorder (ASD). Nevertheless, animal and human studies regarding the link between FMRP and mGluR5 expression provide inconsistent or conflicting findings about the nature of those relationships. Since multiple clinical trials of glutamatergic agents in humans with FXS did not demonstrate the amelioration of the behavioral phenotype observed in animal models of FXS, we sought measure if mGluR5 expression is increased in men with FXS to form the basis for improved clinical trials. Unexpectedly marked reductions in mGluR5 expression were observed in cortical and subcortical regions in men with FXS. Reduced mGluR5 expression throughout the living brains of men with FXS provides a clue to examine FMRP and mGluR5 expression in FXS. In order to develop the findings of our previous study and to strengthen the objective tools for future clinical trials of glutamatergic agents in FXS, we sought to assess the possible value of measuring both FMRP levels and mGluR5 expression in men with FXS. We aimed to show the value of measurement of FMRP levels and mGluR5 expression for the diagnosis and treatment of individuals with FXS and related conditions. We administered 3-[18F]fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB), a specific mGluR5 radioligand for quantitative measurements of the density and the distribution of mGluR5s, to six men with the full mutation (FM) of FXS and to one man with allele size mosaicism for FXS (FXS-M). Utilizing the seven cortical and subcortical regions affected in neurodegenerative disorders as indicator variables, adjusted linear regression of mGluR5 expression and FMRP showed that mGluR5 expression was significantly reduced in the occipital cortex and the thalamus relative to baseline (anterior cingulate cortex) if FMRP levels are held constant (F(7,47) = 6.84, p < 0.001).These findings indicate the usefulness of cerebral mGluR5 expression measured by PET with [18F]FPEB and FMRP values in men with FXS and related conditions for assessments in community facilities within a hundred-mile radius of a production center with a cyclotron. These initial results of this pilot study advance our previous study regarding the measurement of mGluR5 expression by combining both FMRP levels and mGluR5 expression as tools for meaningful clinical trials of glutamatergic agents for men with FXS. We confirm the feasibility of this protocol as a valuable tool to measure FMRP levels and mGluR5 expression in clinical trials of individuals with FXS and related conditions and to provide the foundations to apply precision medicine to tailor treatment plans to the specific needs of individuals with FXS and related conditions.
Keywords: anterior cingulate cortex; correlation coefficient; fragile X mental retardation 1 gene (FMR1); linear regression; neurodevelopmental disorders; neuroimaging; positron emission tomography (PET); radiotracer; temporal cortex; thalamus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Cerebral Expression of Metabotropic Glutamate Receptor Subtype 5 in Idiopathic Autism Spectrum Disorder and Fragile X Syndrome: A Pilot Study.Int J Mol Sci. 2021 Mar 11;22(6):2863. doi: 10.3390/ijms22062863. Int J Mol Sci. 2021. PMID: 33799851 Free PMC article.
-
Reduced Expression of Cerebral Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome.Brain Sci. 2020 Nov 24;10(12):899. doi: 10.3390/brainsci10120899. Brain Sci. 2020. PMID: 33255214 Free PMC article.
-
In vivo imaging of mGlu5 receptor expression in humans with Fragile X Syndrome towards development of a potential biomarker.Sci Rep. 2021 Aug 5;11(1):15897. doi: 10.1038/s41598-021-94967-y. Sci Rep. 2021. PMID: 34354107 Free PMC article.
-
Fragile X syndrome: a preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development.Psychopharmacology (Berl). 2014 Mar;231(6):1217-26. doi: 10.1007/s00213-013-3330-3. Psychopharmacology (Berl). 2014. PMID: 24232444 Review.
-
GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism.Schizophr Res. 2015 Sep;167(1-3):42-56. doi: 10.1016/j.schres.2014.10.010. Epub 2014 Nov 26. Schizophr Res. 2015. PMID: 25432637 Free PMC article. Review.
Cited by
-
Role of mGlu5 in Persistent Forms of Hippocampal Synaptic Plasticity and the Encoding of Spatial Experience.Cells. 2022 Oct 24;11(21):3352. doi: 10.3390/cells11213352. Cells. 2022. PMID: 36359749 Free PMC article. Review.
-
Neurodevelopment and early pharmacological interventions in Fragile X Syndrome.Front Neurosci. 2023 Aug 2;17:1213410. doi: 10.3389/fnins.2023.1213410. eCollection 2023. Front Neurosci. 2023. PMID: 37599992 Free PMC article. Review.
-
The novel peptide LCGM-10 attenuates metabotropic glutamate receptor 5 activity and demonstrates behavioral effects in animal models.Front Behav Neurosci. 2024 Feb 7;18:1333258. doi: 10.3389/fnbeh.2024.1333258. eCollection 2024. Front Behav Neurosci. 2024. PMID: 38385004 Free PMC article.
-
Resting-State Functional MRI and PET Imaging as Noninvasive Tools to Study (Ab)Normal Neurodevelopment in Humans and Rodents.J Neurosci. 2023 Dec 6;43(49):8275-8293. doi: 10.1523/JNEUROSCI.1043-23.2023. J Neurosci. 2023. PMID: 38073598 Free PMC article. Review.
References
-
- Brašić J.R., Nandi A., Russell D.S., Jennings D., Barret O., Mathur A., Slifer K., Sedlak T., Martin S.D., Brinson Z., et al. Reduced Expression of Cerebral Metabotropic Glutamate Receptor Subtype 5 in Men with Fragile X Syndrome. Brain Sci. 2020;10:899. doi: 10.3390/brainsci10120899. - DOI - PMC - PubMed
-
- Budimirovic D.B., Subramanian M. Neurobiology of autism and intellectual disability: Fragile X syndrome. In: Johnston M.V., editor. Neurobiology of Disease. 2nd ed. Oxford University Press; New York, NY, USA: 2016. pp. 375–384.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

