Altered Pain Processing Associated with Administration of Dopamine Agonist and Antagonist in Healthy Volunteers
- PMID: 35326306
- PMCID: PMC8946836
- DOI: 10.3390/brainsci12030351
Altered Pain Processing Associated with Administration of Dopamine Agonist and Antagonist in Healthy Volunteers
Abstract
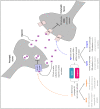
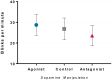
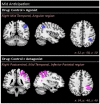
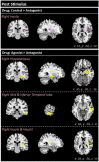
Striatal dopamine dysfunction is associated with the altered top-down modulation of pain processing. The dopamine D2-like receptor family is a potential substrate for such effects due to its primary expression in the striatum, but evidence for this is currently lacking. Here, we investigated the effect of pharmacologically manipulating striatal dopamine D2 receptor activity on the anticipation and perception of acute pain stimuli in humans. Participants received visual cues that induced either certain or uncertain anticipation of two pain intensity levels delivered via a CO2 laser. Rating of the pain intensity and unpleasantness was recorded. Brain activity was recorded with EEG and analysed via source localisation to investigate neural activity during the anticipation and receipt of pain. Participants completed the experiment under three conditions, control (Sodium Chloride), D2 receptor agonist (Cabergoline), and D2 receptor antagonist (Amisulpride), in a repeated-measures, triple-crossover, double-blind study. The antagonist reduced an individuals' ability to distinguish between low and high pain following uncertain anticipation. The EEG source localisation showed that the agonist and antagonist reduced neural activations in specific brain regions associated with the sensory integration of salient stimuli during the anticipation and receipt of pain. During anticipation, the agonist reduced activity in the right mid-temporal region and the right angular gyrus, whilst the antagonist reduced activity within the right postcentral, right mid-temporal, and right inferior parietal regions. In comparison to control, the antagonist reduced activity within the insula during the receipt of pain, a key structure involved in the integration of the sensory and affective aspects of pain. Pain sensitivity and unpleasantness were not changed by D2R modulation. Our results support the notion that D2 receptor neurotransmission has a role in the top-down modulation of pain.
Keywords: D2 receptor; EEG; amisulpride; anticipation; cabergoline; dopamine; pain; source localisation; uncertainty.
Conflict of interest statement
M.A.S. is a member of the editorial board of Brain Sciences. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
No effect of a dopaminergic modulation fMRI task by amisulpride and L-DOPA on reward anticipation in healthy volunteers.Psychopharmacology (Berl). 2021 May;238(5):1333-1342. doi: 10.1007/s00213-020-05693-8. Epub 2020 Nov 2. Psychopharmacology (Berl). 2021. PMID: 33140215 Free PMC article. Clinical Trial.
-
Dopamine-induced changes in neural network patterns supporting aversive conditioning.Brain Res. 2010 Feb 8;1313:143-61. doi: 10.1016/j.brainres.2009.11.064. Epub 2009 Dec 2. Brain Res. 2010. PMID: 19961836 Clinical Trial.
-
Uncertainty in anticipation of uncomfortable rectal distension is modulated by the autonomic nervous system--a fMRI study in healthy volunteers.Neuroimage. 2015 Feb 15;107:10-22. doi: 10.1016/j.neuroimage.2014.11.043. Epub 2014 Dec 3. Neuroimage. 2015. PMID: 25479021
-
Ratio of dopamine synthesis capacity to D2 receptor availability in ventral striatum correlates with central processing of affective stimuli.Eur J Nucl Med Mol Imaging. 2008 Jun;35(6):1147-58. doi: 10.1007/s00259-007-0683-z. Epub 2008 Jan 17. Eur J Nucl Med Mol Imaging. 2008. PMID: 18202844
-
Dopaminergic and serotonergic mechanisms in the modulation of pain: In vivo studies in human brain.Eur J Pharmacol. 2018 Sep 5;834:337-345. doi: 10.1016/j.ejphar.2018.07.038. Epub 2018 Jul 20. Eur J Pharmacol. 2018. PMID: 30036531 Review.
References
-
- Häuser W. Fibromyalgia syndrome: Basic knowledge, diagnosis and treatment. Med. Mon. Pharm. 2016;39:504–511. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

