Opicapone Improves Global Non-Motor Symptoms Burden in Parkinson's Disease: An Open-Label Prospective Study
- PMID: 35326339
- PMCID: PMC8945982
- DOI: 10.3390/brainsci12030383
Opicapone Improves Global Non-Motor Symptoms Burden in Parkinson's Disease: An Open-Label Prospective Study
Abstract
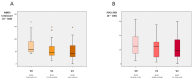
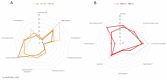
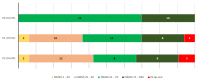
Patients with Parkinson’s disease (PD) can improve some non-motor symptoms (NMS) after starting treatment with opicapone. The aim of this study was to analyze the effectiveness of opicapone on global NMS burden in PD. OPEN-PD (Opicapone Effectiveness on Non-motor symptoms in Parkinson’s Disease) is a prospective open-label single-arm study conducted in 5 centers from Spain. The primary efficacy outcome was the change from baseline (V0) to the end of the observational period (6 months ± 30 days) (V2) in the Non-Motor Symptoms Scale (NMSS) total score. Different scales were used for analyzing the change in motor, NMS, quality of life (QoL), and disability. Thirty-three patients were included between JUL/2019 and JUN/2021 (age 63.3 ± 7.91; 60.6% males; 7.48 ± 4.22 years from symptoms onset). At 6 months, 30 patients completed the follow-up (90.9%). The NMSS total score was reduced by 27.3% (from 71.67 ± 37.12 at V0 to 52.1 ± 34.76 at V2; Cohen’s effect size = −0.97; p = 0.002). By domains, improvement was observed in sleep/fatigue (−40.1%; p < 0.0001), mood/apathy (−46.6%; p = 0.001), gastrointestinal symptoms (−20.7%; p = 0.029), and miscellaneous (−44.94%; p = 0.021). QoL also improved with a 18.4% reduction in the 39-item Parkinson’s Disease Quality of Life Questionnaire Summary Index (from 26.67 ± 17.61 at V0 to 21.75 ± 14.9 at V2; p = 0.001). A total of 13 adverse events in 11 patients (33.3%) were reported, 1 of which was severe (not related to opicapone). Dyskinesias and nausea were the most frequent (6.1%). Opicapone is well tolerated and improves global NMS burden and QoL in PD patients at 6 months.
Keywords: Parkinson’s disease; effectiveness; non-motor symptoms; open-label study; opicapone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Adverse event profiles of adjuvant treatment with opicapone in Parkinson's disease: A systematic review and meta-analysis.Front Pharmacol. 2022 Nov 24;13:1042992. doi: 10.3389/fphar.2022.1042992. eCollection 2022. Front Pharmacol. 2022. PMID: 36506576 Free PMC article.
-
Safinamide Improves Non-Motor Symptoms Burden in Parkinson's Disease: An Open-Label Prospective Study.Brain Sci. 2021 Mar 2;11(3):316. doi: 10.3390/brainsci11030316. Brain Sci. 2021. PMID: 33801565 Free PMC article.
-
Opicapone for Parkinson's disease-related sleep disturbances: The OASIS clinical trial.J Parkinsons Dis. 2025 Feb;15(1):87-96. doi: 10.1177/1877718X241306711. Epub 2025 Jan 17. J Parkinsons Dis. 2025. PMID: 39973499 Clinical Trial.
-
Effectiveness and safety of opicapone in Parkinson's disease patients with motor fluctuations: the OPTIPARK open-label study.Transl Neurodegener. 2020 Mar 4;9(1):9. doi: 10.1186/s40035-020-00187-1. Transl Neurodegener. 2020. PMID: 32345378 Free PMC article. Clinical Trial.
-
Effects of Levodopa-Carbidopa Intestinal Gel on Dyskinesia and Non-Motor Symptoms Including Sleep: Results from a Meta-Analysis with 24-Month Follow-Up.J Parkinsons Dis. 2022;12(7):2071-2083. doi: 10.3233/JPD-223295. J Parkinsons Dis. 2022. PMID: 35964203 Free PMC article. Review.
Cited by
-
Adverse event profiles of adjuvant treatment with opicapone in Parkinson's disease: A systematic review and meta-analysis.Front Pharmacol. 2022 Nov 24;13:1042992. doi: 10.3389/fphar.2022.1042992. eCollection 2022. Front Pharmacol. 2022. PMID: 36506576 Free PMC article.
-
Motor Fluctuations Development Is Associated with Non-Motor Symptoms Burden Progression in Parkinson's Disease Patients: A 2-Year Follow-Up Study.Diagnostics (Basel). 2022 May 5;12(5):1147. doi: 10.3390/diagnostics12051147. Diagnostics (Basel). 2022. PMID: 35626303 Free PMC article.
-
Levodopa-Carbidopa Intestinal Gel in Advanced Parkinson's Disease: Observations and Dilemmas after 10 Years of Real-Life Experience.Pharmaceutics. 2022 May 24;14(6):1115. doi: 10.3390/pharmaceutics14061115. Pharmaceutics. 2022. PMID: 35745688 Free PMC article.
-
Non-Motor Fluctuations in Parkinson's Disease: Underdiagnosed Yet Important.J Mov Disord. 2025 Jan;18(1):1-16. doi: 10.14802/jmd.24227. Epub 2024 Dec 20. J Mov Disord. 2025. PMID: 39703981 Free PMC article.
-
Clinical benefit of MAO-B and COMT inhibition in Parkinson's disease: practical considerations.J Neural Transm (Vienna). 2023 Jun;130(6):847-861. doi: 10.1007/s00702-023-02623-8. Epub 2023 Mar 24. J Neural Transm (Vienna). 2023. PMID: 36964457 Free PMC article. Review.
References
-
- Santos García D., de Deus Fonticoba T., Suárez Castro E., Borrué C., Mata M., Solano Vila B., Cots Foraster A., Álvarez Sauco M., Coppadis Study Group Non-motor symptoms burden, mood, and gait problems are the most significant factors contributing to a poor quality of life in non-demented Parkinson’s disease patients: Results from the COPPADIS Study Cohort. Parkinsonism Relat. Disord. 2019;66:151–157. doi: 10.1016/j.parkreldis.2019.07.031. - DOI - PubMed
-
- Seppi K., Weintraub D., Coelho M., Perez-Lloret S., Fox S.H., Katzenschlager R., Hametner E.-M., Poewe W., Rascol O., Goetz C.G., et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motorsymptoms of Parkinson’s disease. Mov. Disord. 2011;26((Suppl. 3)):S42–S80. doi: 10.1002/mds.23884. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

