New Insight in Hyperinsulinism/Hyperammonemia Syndrome by Magnetic Resonance Imaging and Spectroscopy
- PMID: 35326344
- PMCID: PMC8946637
- DOI: 10.3390/brainsci12030389
New Insight in Hyperinsulinism/Hyperammonemia Syndrome by Magnetic Resonance Imaging and Spectroscopy
Abstract
Hyperinsulinism/hyperammonemia syndrome (HI/HA) is an autosomal dominant disorder caused by monoallelic activating mutations in the glutamate dehydrogenase 1 (GLUD1) gene. While hyperinsulinism may be explained by a reduction in the allosteric inhibition of GLUD1, the pathogenesis of HA in HI/HA remains uncertain; interestingly, HA in the HI/HA syndrome is not associated with acute hyperammonemic intoxication events. We obtained a brain magnetic resonance (MR) in a woman with HI/HA syndrome with chronic asymptomatic HA. On MR spectroscopy, choline and myoinositol were decreased as in other HA disorders. In contrast, distinct from other HA disorders, combined glutamate and glutamine levels were normal (not increased). This observation suggests that brain biochemistry in HI/HA may differ from that of other HA disorders. In HI/HA, ammonia overproduction may come to the expense of glutamate levels, and this seems to prevent the condensation of ammonia with glutamate to produce glutamine that is typical of the other HA disorders. The absence of combined glutamate and glutamine elevation might be correlated to the absence of acute cerebral ammonia toxicity.
Keywords: brain spectroscopy; glutamate dehydrogenase; hyperammonemia; hyperinsulinism.
Conflict of interest statement
The authors declared no potential conflict of interest.
Figures
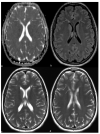
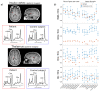
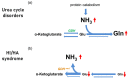
References
-
- Stanley C.A., Lieu Y.K., Hsu B.Y., Burlina A.B., Greenberg C.R., Hopwood N.J., Perlman K., Rich B.H., Zammarchi E., Poncz M. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N. Engl. J. Med. 1998;338:1352–1357. doi: 10.1056/NEJM199805073381904. - DOI - PubMed
-
- Bahi-Buisson N., Roze E., Dionisi C., Escande F., Valayannopoulos V., Feillet F., Heinrichs C., Chadefaux-Vekemans B., Dan B., de Lonlay P. Neurological aspects of hyperinsulinism-hyperammonaemia syndrome. Dev. Med. Child Neurol. 2008;50:945–949. doi: 10.1111/j.1469-8749.2008.03114.x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

