Cytochrome c Oxidase Activity as a Metabolic Regulator in Pancreatic Beta-Cells
- PMID: 35326380
- PMCID: PMC8946064
- DOI: 10.3390/cells11060929
Cytochrome c Oxidase Activity as a Metabolic Regulator in Pancreatic Beta-Cells
Abstract
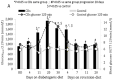
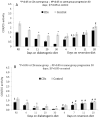
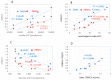
Pancreatic β-cells couple glucose-stimulated insulin secretion (GSIS) with oxidative phosphorylation via cytochrome c oxidase (COX), a mitochondrial respiratory-chain enzyme. The Cohen diabetic-sensitive (CDs) rats exhibit hyperglycemia when fed a diabetogenic diet but maintain normoglycemia on a regular diet. We have previously reported a decreased COX activity in CDs rats and explored its relevance for type 2 diabetes (T2D). In this study, we investigated the relation between COX activity in islets, peripheral-blood mononuclear cells (PBMCs), and GSIS during diabetes development in CDs rats fed a diabetogenic diet for 4, 11, 20, and 30 days and during reversion to normoglycemia in hyperglycemic CDs rats fed a reversion diet for 7, 11, and 20 days. An oral glucose-tolerance test was performed at different periods of the diets measuring blood glucose and insulin concentrations. COX activity was determined in islets and PBMCs isolated from rats at the different periods of the diets. We demonstrated a progressive reduction in COX activity in CDs-islets that correlated positively with the decreasing GSIS (R2 = 0.9691, p < 0.001) and inversely with the elevation in blood glucose levels (R2 = 0.8396, p < 0.001). Hyperglycemia was initiated when islet COX activity decreased below 46%. The reversion diet restored >46% of the islet COX activity and GSIS while re-establishing normoglycemia. Interestingly, COX activity in PBMCs correlated significantly with islet COX activity (R2 = 0.8944, p < 0.001). Our data support islet COX activity as a major metabolic regulator of β-cells function. The correlation between COX activity in PBMCs and islets may serve as a noninvasive biomarker to monitor β-cell dysfunction in diabetes.
Keywords: blood glucose levels; cytochrome c oxidase; glucose stimulated insulin secretion; pancreatic β-cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Parnis J., Rutter G.A. Contributions of Mitochondrial Dysfunction to β Cell Failure in Diabetes Mellitus. In: Morio B., Pénicaud L., Rigoulet M., editors. Mitochondria in Obesity and Type 2 Diabetes. Elsevier Inc.; Amsterdam, The Netherlands: 2019.
-
- Zhang E., Mohammed Al-Amily I., Mohammed S., Luan C., Asplund O., Ahmed M., Ye Y., Ben-Hail D., Soni A., Vishnu N., et al. Preserving Insulin Secretion in Diabetes by Inhibiting VDAC1 Overexpression and Surface Translocation in β Cells. Cell Metab. 2018;29:64–77.e6. doi: 10.1016/j.cmet.2018.09.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

