HPLC-PDA-ESI-HRMS-Based Profiling of Secondary Metabolites of Rindera graeca Anatomical and Hairy Roots Treated with Drought and Cold Stress
- PMID: 35326382
- PMCID: PMC8946546
- DOI: 10.3390/cells11060931
HPLC-PDA-ESI-HRMS-Based Profiling of Secondary Metabolites of Rindera graeca Anatomical and Hairy Roots Treated with Drought and Cold Stress
Abstract
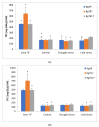
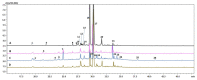
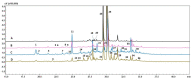
To cope with environmental harmful conditions, plant cells developed adaptive strategy that involves production of a wide variety of complex secondary metabolites. The spectrum and quantity of biosynthesized compounds in specific plant species is determined by its genotype, tissue, developmental and physiological stage and environmental factors. This phenomenon was used to exploit the potential of anatomical and hairy root cultures of Rindera graeca to produce bioactive compounds. Cultivated in vitro roots were subjected to abiotic stresses i.e., drought or coldness. Next the extract profiling was performed using HPLC-PDA-ESI-HRMS method, as well quantitative determination of caffeic, rosmarinic and lithospermic B acids, that were present in all root extracts. Phenolic acids, flavonoids and iridoids represent the major groups of compounds detected in chemical profiles growing under various conditions roots. The highest number of phytochemicals was determined in roots subjected to coldness. Lithospermic B acid proved to be the most abundant compound in all investigated extracts. Among applied abiotic stress factors it was demonstrated that coldness affected to the most secondary metabolites production. The results of current study suggest that root cultures of R. graeca could serve as a new and abundant source of lithospermic B acid.
Keywords: Boraginaceae rosmarinic acid; abiotic stresses; chemical profile; in vitro cultures; lithospermic B acid; total phenolic and total flavonoid content.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Rindera graeca (A. DC.) Boiss. & Heldr. (Boraginaceae) In Vitro Cultures Targeting Lithospermic Acid B and Rosmarinic Acid Production.Molecules. 2023 Jun 20;28(12):4880. doi: 10.3390/molecules28124880. Molecules. 2023. PMID: 37375435 Free PMC article.
-
Rindera graeca (Boraginaceae) Phytochemical Profile and Biological Activities.Molecules. 2020 Aug 9;25(16):3625. doi: 10.3390/molecules25163625. Molecules. 2020. PMID: 32784926 Free PMC article.
-
Chemical Profile and Screening of Bioactive Metabolites of Rindera graeca (A. DC.) Bois. & Heldr. (Boraginaceae) In Vitro Cultures.Plants (Basel). 2021 Apr 21;10(5):834. doi: 10.3390/plants10050834. Plants (Basel). 2021. PMID: 33919433 Free PMC article.
-
Hairy root culture for mass-production of high-value secondary metabolites.Crit Rev Biotechnol. 2007 Jan-Mar;27(1):29-43. doi: 10.1080/07388550601173918. Crit Rev Biotechnol. 2007. PMID: 17364688 Review.
-
Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation.Microbiol Res. 2021 Jan;242:126626. doi: 10.1016/j.micres.2020.126626. Epub 2020 Oct 18. Microbiol Res. 2021. PMID: 33189069 Review.
Cited by
-
Hairy Root Cultures as a Source of Phenolic Antioxidants: Simple Phenolics, Phenolic Acids, Phenylethanoids, and Hydroxycinnamates.Int J Mol Sci. 2023 Apr 7;24(8):6920. doi: 10.3390/ijms24086920. Int J Mol Sci. 2023. PMID: 37108084 Free PMC article. Review.
-
UHPLC-MS Phenolic Fingerprinting, Aorta Endothelium Relaxation Effect, Antioxidant, and Enzyme Inhibition Activities of Azara dentata Ruiz & Pav Berries.Foods. 2023 Feb 2;12(3):643. doi: 10.3390/foods12030643. Foods. 2023. PMID: 36766170 Free PMC article.
-
Rindera graeca (A. DC.) Boiss. & Heldr. (Boraginaceae) In Vitro Cultures Targeting Lithospermic Acid B and Rosmarinic Acid Production.Molecules. 2023 Jun 20;28(12):4880. doi: 10.3390/molecules28124880. Molecules. 2023. PMID: 37375435 Free PMC article.
-
Multifaceted insights into the environmental adaptability of Arnebia guttata under drought stress.Front Plant Sci. 2024 Jun 13;15:1395046. doi: 10.3389/fpls.2024.1395046. eCollection 2024. Front Plant Sci. 2024. PMID: 38938629 Free PMC article.
-
Computational Approach for the Development of pH-Selective PD-1/PD-L1 Signaling Pathway Inhibition in Fight with Cancer.Cancers (Basel). 2024 Jun 22;16(13):2295. doi: 10.3390/cancers16132295. Cancers (Basel). 2024. PMID: 39001358 Free PMC article.
References
-
- Kamari G., Blanché C., Siljak-Yakovlev S. Mediterranean chromosome number reports—24. Flora Mediterr. 2014;24:286–291. doi: 10.7320/FlMedit24.273. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

