Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients
- PMID: 35326383
- PMCID: PMC8946177
- DOI: 10.3390/cells11060932
Hyperglycemia and Loss of Redox Homeostasis in COVID-19 Patients
Abstract
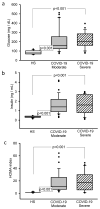
The infection with SARS-CoV-2 impairs the glucose−insulin axis and this contributes to oxidative (OS) and nitrosative (NSS) stress. Here, we evaluated changes in glucose metabolism that could promote the loss of redox homeostasis in COVID-19 patients. This was comparative cohort and analytical study that compared COVID-19 patients and healthy subjects. The study population consisted of 61 COVID-19 patients with and without comorbidities and 25 healthy subjects (HS). In all subjects the plasma glucose, insulin, 8-isoprostane, Vitamin D, H2S and 3-nitrotyrosine were determined by ELISA. The nitrites (NO2−), lipid-peroxidation (LPO), total-antioxidant-capacity (TAC), thiols, glutathione (GSH) and selenium (Se) were determined by spectrophotometry. The glucose, insulin and HOMA-IR (p < 0.001), 8-isoprostanes, 3-nitrotyrosine (p < 0.001) and LPO were increased (p = 0.02) while Vitamin D (p = 0.01), H2S, thiols, TAC, GSH and Se (p < 0.001) decreased in COVID-19 patients in comparison to HS. The SARS-CoV-2 infection resulted in alterations in the glucose−insulin axis that led to hyperglycemia, hyperinsulinemia and IR in patients with and without comorbidities. These alterations increase OS and NSS reflected in increases or decreases in some oxidative markers in plasma with major impact or fatal consequences in patients that course with metabolic syndrome. Moreover, subjects without comorbidities could have long-term alterations in the redox homeostasis after infection.
Keywords: H2S; SARS-CoV-2; homeostasis redox; hyperglycemia; lipid peroxidation; nitrotyrosine; selenium; thiols.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Redox Homeostasis Alteration Is Restored through Melatonin Treatment in COVID-19 Patients: A Preliminary Study.Int J Mol Sci. 2024 Apr 21;25(8):4543. doi: 10.3390/ijms25084543. Int J Mol Sci. 2024. PMID: 38674128 Free PMC article.
-
N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings.Pharmaceuticals (Basel). 2023 Apr 14;16(4):591. doi: 10.3390/ph16040591. Pharmaceuticals (Basel). 2023. PMID: 37111348 Free PMC article.
-
Oxidative status and its relation with insulin resistance in young non-obese women with polycystic ovary syndrome.J Endocrinol Invest. 2012 Mar;35(3):317-21. doi: 10.3275/7682. Epub 2011 Apr 26. J Endocrinol Invest. 2012. PMID: 21521935
-
Role of selenium toxicity and oxidative stress in aquatic birds.Aquat Toxicol. 2002 Apr;57(1-2):11-26. doi: 10.1016/s0166-445x(01)00263-6. Aquat Toxicol. 2002. PMID: 11879935 Review.
-
Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism.Free Radic Biol Med. 2013 Dec;65:1538-1547. doi: 10.1016/j.freeradbiomed.2013.07.016. Epub 2013 Jul 18. Free Radic Biol Med. 2013. PMID: 23872396 Review.
Cited by
-
Obesity as a Risk Factor for the Severity of COVID-19 in Pediatric Patients: Possible Mechanisms-A Narrative Review.Children (Basel). 2024 Sep 30;11(10):1203. doi: 10.3390/children11101203. Children (Basel). 2024. PMID: 39457167 Free PMC article. Review.
-
The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, Long COVID, and ME/CFS: evidence, mechanisms, and therapeutic implications.Biochem J. 2022 Aug 31;479(16):1653-1708. doi: 10.1042/BCJ20220154. Biochem J. 2022. PMID: 36043493 Free PMC article. Review.
-
Diversified Effects of COVID-19 as a Consequence of the Differential Metabolism of Phospholipids and Lipid Peroxidation Evaluated in the Plasma of Survivors and Deceased Patients upon Admission to the Hospital.Int J Mol Sci. 2022 Oct 5;23(19):11810. doi: 10.3390/ijms231911810. Int J Mol Sci. 2022. PMID: 36233111 Free PMC article.
-
Severe COVID-19 associated hyperglycemia is caused by beta cell dysfunction: a prospective cohort study.Nutr Diabetes. 2023 Jul 17;13(1):11. doi: 10.1038/s41387-023-00241-7. Nutr Diabetes. 2023. PMID: 37460458 Free PMC article.
-
Exosomal miR-145 and miR-885 Regulate Thrombosis in COVID-19.J Pharmacol Exp Ther. 2023 Jan;384(1):109-115. doi: 10.1124/jpet.122.001209. Epub 2022 Jun 30. J Pharmacol Exp Ther. 2023. PMID: 35772782 Free PMC article.
References
-
- Soria-Castro E., Soto M.E., Guarner-Lans V., Rojas G., Perezpeña-Diazconti M., Críales-Vera S.A., Manzano-Pech L., Pérez-Torres I. The kidnapping of mitochondrial function associated with the SARS-CoV-2 infection. Histol. Histopathol. 2021;36:947–965. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

