Breast Cancer Stem Cell Membrane Biomarkers: Therapy Targeting and Clinical Implications
- PMID: 35326385
- PMCID: PMC8946706
- DOI: 10.3390/cells11060934
Breast Cancer Stem Cell Membrane Biomarkers: Therapy Targeting and Clinical Implications
Abstract
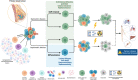
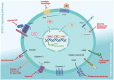
Breast cancer is the most common malignancy affecting women worldwide. Importantly, there have been significant improvements in prevention, early diagnosis, and treatment options, which resulted in a significant decrease in breast cancer mortality rates. Nevertheless, the high rates of incidence combined with therapy resistance result in cancer relapse and metastasis, which still contributes to unacceptably high mortality of breast cancer patients. In this context, a small subpopulation of highly tumourigenic cancer cells within the tumour bulk, commonly designated as breast cancer stem cells (BCSCs), have been suggested as key elements in therapy resistance, which are responsible for breast cancer relapses and distant metastasis. Thus, improvements in BCSC-targeting therapies are crucial to tackling the metastatic progression and might allow therapy resistance to be overcome. However, the design of effective and specific BCSC-targeting therapies has been challenging since there is a lack of specific biomarkers for BCSCs, and the most common clinical approaches are designed for commonly altered BCSCs signalling pathways. Therefore, the search for a new class of BCSC biomarkers, such as the expression of membrane proteins with cancer stem cell potential, is an area of clinical relevance, once membrane proteins are accessible on the cell surface and easily recognized by specific antibodies. Here, we discuss the significance of BCSC membrane biomarkers as potential prognostic and therapeutic targets, reviewing the CSC-targeting therapies under clinical trials for breast cancer.
Keywords: breast cancer stem cells; cell membrane biomarkers; targeted therapies; translational oncobiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications.Pharmacol Res. 2021 Jan;163:105320. doi: 10.1016/j.phrs.2020.105320. Epub 2020 Dec 1. Pharmacol Res. 2021. PMID: 33271295 Review.
-
Cancer stem cell in breast cancer therapeutic resistance.Cancer Treat Rev. 2018 Sep;69:152-163. doi: 10.1016/j.ctrv.2018.07.004. Epub 2018 Jul 18. Cancer Treat Rev. 2018. PMID: 30029203 Review.
-
Breast cancer stem cell: the roles and therapeutic implications.Cell Mol Life Sci. 2017 Mar;74(6):951-966. doi: 10.1007/s00018-016-2334-7. Epub 2016 Aug 16. Cell Mol Life Sci. 2017. PMID: 27530548 Free PMC article. Review.
-
Transcriptomic but not genomic variability confers phenotype of breast cancer stem cells.Cancer Commun (Lond). 2018 Sep 19;38(1):56. doi: 10.1186/s40880-018-0326-8. Cancer Commun (Lond). 2018. PMID: 30231942 Free PMC article.
-
Elimination of epithelial-like and mesenchymal-like breast cancer stem cells to inhibit metastasis following nanoparticle-mediated photothermal therapy.Biomaterials. 2016 Oct;104:145-57. doi: 10.1016/j.biomaterials.2016.06.045. Epub 2016 Jun 23. Biomaterials. 2016. PMID: 27450902 Free PMC article.
Cited by
-
Genetic evidence supporting potential causal roles of EIF4 family in breast cancer: a two-sample randomized Mendelian study.Sci Rep. 2024 Aug 30;14(1):20191. doi: 10.1038/s41598-024-71059-1. Sci Rep. 2024. PMID: 39215053 Free PMC article.
-
Impact of prolactin treatment on enhancing the cellular responses of MCF7 breast cancer cells to tamoxifen treatment.Discov Oncol. 2024 Dec 18;15(1):797. doi: 10.1007/s12672-024-01701-x. Discov Oncol. 2024. PMID: 39692941 Free PMC article.
-
C1ql4 regulates breast cancer cell stemness and epithelial-mesenchymal transition through PI3K/AKT/NF-κB signaling pathway.Front Oncol. 2023 May 31;13:1192482. doi: 10.3389/fonc.2023.1192482. eCollection 2023. Front Oncol. 2023. PMID: 37324011 Free PMC article.
-
Mapping Knowledge Landscapes and Themes Trends of Breast Cancer Stem Cells: A Comprehensive Data-Mining-Based Study.Ann Surg Oncol. 2025 Aug;32(8):6031-6049. doi: 10.1245/s10434-025-17336-3. Epub 2025 Apr 23. Ann Surg Oncol. 2025. PMID: 40268846
-
Preclinical and Clinical Trials of New Treatment Strategies Targeting Cancer Stem Cells in Subtypes of Breast Cancer.Cells. 2023 Feb 24;12(5):720. doi: 10.3390/cells12050720. Cells. 2023. PMID: 36899854 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

