Differential Involvement of Arabidopsis β'-COP Isoforms in Plant Development
- PMID: 35326389
- PMCID: PMC8946003
- DOI: 10.3390/cells11060938
Differential Involvement of Arabidopsis β'-COP Isoforms in Plant Development
Abstract
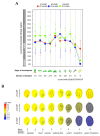
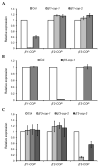
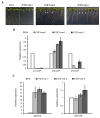
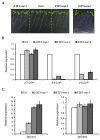
Coat protein I (COPI) is necessary for intra-Golgi transport and retrograde transport from the Golgi apparatus back to the endoplasmic reticulum. The key component of the COPI coat is the coatomer complex, which is composed of seven subunits (α/β/β'/γ/δ/ε/ζ) and is recruited en bloc from the cytosol onto Golgi membranes. In mammals and yeast, α- and β'-COP WD40 domains mediate cargo-selective interactions with dilysine motifs present in canonical cargoes of COPI vesicles. In contrast to mammals and yeast, three isoforms of β'-COP (β'1-3-COP) have been identified in Arabidopsis. To understand the role of Arabidopsis β'-COP isoforms in plant biology, we have identified and characterized loss-of-function mutants of the three isoforms, and double mutants were also generated. We have found that the trafficking of a canonical dilysine cargo (the p24 family protein p24δ5) is affected in β'-COP double mutants. By western blot analysis, it is also shown that protein levels of α-COP are reduced in the β'-COP double mutants. Although none of the single mutants showed an obvious growth defect, double mutants showed different growth phenotypes. The double mutant analysis suggests that, under standard growth conditions, β'1-COP can compensate for the loss of both β'2-COP and β'3-COP and may have a prominent role during seedling development.
Keywords: Arabidopsis; coat protein I (COPI); isoforms; plant growth; α-COP; β’-COP.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
The presence of β'1-COP and β'2-COP is required for female and male gametophyte development.Plant Reprod. 2023 Dec;36(4):343-347. doi: 10.1007/s00497-023-00467-6. Epub 2023 Jun 2. Plant Reprod. 2023. PMID: 37266760 Free PMC article.
-
α2-COP is involved in early secretory traffic in Arabidopsis and is required for plant growth.J Exp Bot. 2017 Jan 1;68(3):391-401. doi: 10.1093/jxb/erw446. J Exp Bot. 2017. PMID: 28025315 Free PMC article.
-
The alpha- and beta'-COP WD40 domains mediate cargo-selective interactions with distinct di-lysine motifs.Mol Biol Cell. 2004 Mar;15(3):1011-23. doi: 10.1091/mbc.e03-10-0724. Epub 2003 Dec 29. Mol Biol Cell. 2004. PMID: 14699056 Free PMC article.
-
Loss of Arabidopsis β-COP Function Affects Golgi Structure, Plant Growth and Tolerance to Salt Stress.Front Plant Sci. 2020 Apr 15;11:430. doi: 10.3389/fpls.2020.00430. eCollection 2020. Front Plant Sci. 2020. PMID: 32351533 Free PMC article.
-
COPs regulating membrane traffic.Annu Rev Cell Dev Biol. 1995;11:677-706. doi: 10.1146/annurev.cb.11.110195.003333. Annu Rev Cell Dev Biol. 1995. PMID: 8689572 Review.
Cited by
-
Arabidopsis TETRASPANIN8 mediates exosome secretion and glycosyl inositol phosphoceramide sorting and trafficking.Plant Cell. 2024 Feb 26;36(3):626-641. doi: 10.1093/plcell/koad285. Plant Cell. 2024. PMID: 37950906 Free PMC article.
-
The presence of β'1-COP and β'2-COP is required for female and male gametophyte development.Plant Reprod. 2023 Dec;36(4):343-347. doi: 10.1007/s00497-023-00467-6. Epub 2023 Jun 2. Plant Reprod. 2023. PMID: 37266760 Free PMC article.
References
-
- Shomron O., Nevo-Yassaf I., Aviad T., Yaffe Y., Zahavi E.E., Dukhovny A., Perlson E., Brodsky I., Yeheskel A., Pasmanik-Chor M., et al. COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers. J. Cell Biol. 2021;220:e201907224. doi: 10.1083/jcb.201907224. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

