Vav1 Promotes B-Cell Lymphoma Development
- PMID: 35326399
- PMCID: PMC8946024
- DOI: 10.3390/cells11060949
Vav1 Promotes B-Cell Lymphoma Development
Abstract
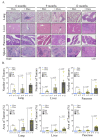
Vav1 is normally and exclusively expressed in the hematopoietic system where it functions as a specific GDP/GTP nucleotide exchange factor (GEF), firmly regulated by tyrosine phosphorylation. Mutations and overexpression of Vav1 in hematopoietic malignancies, and in human cancers of various histologic origins, are well documented. To reveal whether overexpression of Vav1 in different tissues suffices for promoting the development of malignant lesions, we expressed Vav1 in transgenic mice by using the ubiquitous ROSA26 promoter (Rosa Vav1). We detected Vav1 expression in epithelial tissues of various organs including pancreas, liver, and lung. While carcinomas did not develop in these organs, surprisingly, we noticed the development of B-cell lymphomas. Rac1-GTP levels did not change in tissues from Rosa Vav1 mice expressing the transgenic Vav1, while ERK phosphorylation increased in the lymphomas, suggesting that signaling pathways are evoked. One of the growth factors analyzed by us as a suspect candidate to mediate paracrine stimulation in the lymphocytes was CSF-1, which was highly expressed in the epithelial compartment of Rosa Vav1 mice. The expression of its specific receptor, CSF-1R, was found to be highly expressed in the B-cell lymphomas. Taken together, our results suggest a potential cross-talk between epithelial cells expressing Vav1, that secrete CSF-1, and the lymphocytes that express CSF-1R, thus leading to the generation of B-cell lymphomas. Our findings provide a novel mechanism by which Vav1 contributes to tumor propagation.
Keywords: B-cell lymphoma; Rac-GTP; Rosa26; Vav1.
Conflict of interest statement
The authors have no competing interests to declare.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

