Spatial Manipulation of Particles and Cells at Micro- and Nanoscale via Magnetic Forces
- PMID: 35326401
- PMCID: PMC8946034
- DOI: 10.3390/cells11060950
Spatial Manipulation of Particles and Cells at Micro- and Nanoscale via Magnetic Forces
Abstract
The importance of magnetic micro- and nanoparticles for applications in biomedical technology is widely recognised. Many of these applications, including tissue engineering, cell sorting, biosensors, drug delivery, and lab-on-chip devices, require remote manipulation of magnetic objects. High-gradient magnetic fields generated by micromagnets in the range of 103-105 T/m are sufficient for magnetic forces to overcome other forces caused by viscosity, gravity, and thermal fluctuations. In this paper, various magnetic systems capable of generating magnetic fields with required spatial gradients are analysed. Starting from simple systems of individual magnets and methods of field computation, more advanced magnetic microarrays obtained by lithography patterning of permanent magnets are introduced. More flexible field configurations can be formed with the use of soft magnetic materials magnetised by an external field, which allows control over both temporal and spatial field distributions. As an example, soft magnetic microwires are considered. A very attractive method of field generation is utilising tuneable domain configurations. In this review, we discuss the force requirements and constraints for different areas of application, emphasising the current challenges and how to overcome them.
Keywords: cell arraying; diamagnetic levitation; gradient magnetic field; magnetic force; micromagnetic array.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

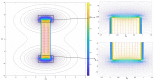


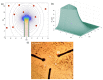

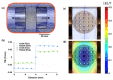

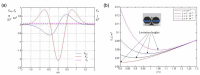
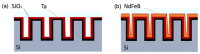


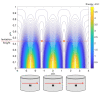



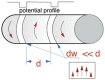

References
-
- Cao Q.L., Fan Q., Chen Q., Liu C.T., Han X.T., Li L. Recent advances in manipulation of micro- and nano-objects with magnetic fields at small scales. Mater. Horiz. 2020;7:638–666. doi: 10.1039/C9MH00714H. - DOI
-
- Rajan A., Sahu N.K. Review on magnetic nanoparticle-mediated hyperthermia for cancer therapy. J. Nanopart. Res. 2020;22:319. doi: 10.1007/s11051-020-05045-9. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

