Palmitate-Triggered COX2/PGE2-Related Hyperinflammation in Dual-Stressed PdL Fibroblasts Is Mediated by Repressive H3K27 Trimethylation
- PMID: 35326406
- PMCID: PMC8946768
- DOI: 10.3390/cells11060955
Palmitate-Triggered COX2/PGE2-Related Hyperinflammation in Dual-Stressed PdL Fibroblasts Is Mediated by Repressive H3K27 Trimethylation
Abstract
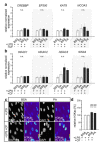
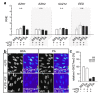
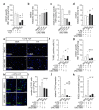
The interrelationships between periodontal disease, obesity-related hyperlipidemia and mechanical forces and their modulating effects on the epigenetic profile of periodontal ligament (PdL) cells are assumed to be remarkably complex. The PdL serves as a connective tissue between teeth and alveolar bone and is involved in pathogen defense and the inflammatory responses to mechanical stimuli occurring during tooth movement. Altered inflammatory signaling could promote root resorption and tooth loss. Hyperinflammatory COX2/PGE2 signaling was reported for human PdL fibroblasts (HPdLFs) concomitantly stressed with Porphyromonas gingivalis lipopolysaccharides and compressive force after exposure to palmitic acid (PA). The aim of this study was to investigate the extent to which this was modulated by global and gene-specific changes in histone modifications. The expression of key epigenetic players and global H3Kac and H3K27me3 levels were quantitatively evaluated in dual-stressed HPdLFs exposed to PA, revealing a minor force-related reduction in repressive H3K27me3. UNC1999-induced H3K27me3 inhibition reversed the hyperinflammatory responses of dual-stressed PA cultures characterized by increased COX2 expression, PGE2 secretion and THP1 adhesion. The reduced expression of the gene encoding the anti-inflammatory cytokine IL-10 and the increased presence of H3K27me3 at its promoter-associated sites were reversed by inhibitor treatment. Thus, the data highlight an important epigenetic interplay between the different stimuli to which the PdL is exposed.
Keywords: COX2/PGE2; IL-10; histone modification; inflammation; obesity; palmitic acid; periodontitis; tooth movement.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Oleic acid-related anti-inflammatory effects in force-stressed PdL fibroblasts are mediated by H3 lysine acetylation associated with altered IL10 expression.Epigenetics. 2022 Dec;17(13):1892-1904. doi: 10.1080/15592294.2022.2090654. Epub 2022 Jun 28. Epigenetics. 2022. PMID: 35763686 Free PMC article.
-
GDF15 Modulates the Zoledronic-Acid-Induced Hyperinflammatory Mechanoresponse of Periodontal Ligament Fibroblasts.Cells. 2024 Jan 12;13(2):147. doi: 10.3390/cells13020147. Cells. 2024. PMID: 38247838 Free PMC article.
-
Hyperlipidemic Conditions Impact Force-Induced Inflammatory Response of Human Periodontal Ligament Fibroblasts Concomitantly Challenged with P. gingivalis-LPS.Int J Mol Sci. 2021 Jun 4;22(11):6069. doi: 10.3390/ijms22116069. Int J Mol Sci. 2021. PMID: 34199865 Free PMC article.
-
GDF15 Supports the Inflammatory Response of PdL Fibroblasts Stimulated by P. gingivalis LPS and Concurrent Compression.Int J Mol Sci. 2021 Dec 19;22(24):13608. doi: 10.3390/ijms222413608. Int J Mol Sci. 2021. PMID: 34948405 Free PMC article.
-
Mechanisms involved in regulation of periodontal ligament cell production of pro-inflammatory cytokines: Implications in periodontitis.J Periodontal Res. 2021 Apr;56(2):249-255. doi: 10.1111/jre.12823. Epub 2020 Dec 10. J Periodontal Res. 2021. PMID: 33305420 Free PMC article. Review.
Cited by
-
GDF15 Promotes the Osteogenic Cell Fate of Periodontal Ligament Fibroblasts, thus Affecting Their Mechanobiological Response.Int J Mol Sci. 2023 Jun 11;24(12):10011. doi: 10.3390/ijms241210011. Int J Mol Sci. 2023. PMID: 37373159 Free PMC article.
-
Oleic acid-related anti-inflammatory effects in force-stressed PdL fibroblasts are mediated by H3 lysine acetylation associated with altered IL10 expression.Epigenetics. 2022 Dec;17(13):1892-1904. doi: 10.1080/15592294.2022.2090654. Epub 2022 Jun 28. Epigenetics. 2022. PMID: 35763686 Free PMC article.
-
GDF15 Modulates the Zoledronic-Acid-Induced Hyperinflammatory Mechanoresponse of Periodontal Ligament Fibroblasts.Cells. 2024 Jan 12;13(2):147. doi: 10.3390/cells13020147. Cells. 2024. PMID: 38247838 Free PMC article.
-
Saturated fatty acids negatively affect musculoskeletal tissues in vitro and in vivo.Matrix Biol Plus. 2024 May 31;23:100153. doi: 10.1016/j.mbplus.2024.100153. eCollection 2024 Aug. Matrix Biol Plus. 2024. PMID: 38882396 Free PMC article.
-
Dual Roles of Prostaglandin E2 (PGE2) in Bone Remodeling and Pain Management: Bridging the Gap in Osteoarthritis Research.Mediators Inflamm. 2025 Jul 26;2025:8882429. doi: 10.1155/mi/8882429. eCollection 2025. Mediators Inflamm. 2025. PMID: 40757049 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

