Methodological Flaws in Meta-Analyses of Clinical Studies on the Management of Knee Osteoarthritis with Stem Cells: A Systematic Review
- PMID: 35326416
- PMCID: PMC8946093
- DOI: 10.3390/cells11060965
Methodological Flaws in Meta-Analyses of Clinical Studies on the Management of Knee Osteoarthritis with Stem Cells: A Systematic Review
Abstract
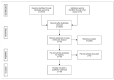
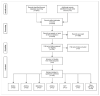
(1) Background: Conclusions of meta-analyses of clinical studies may substantially influence opinions of prospective patients and stakeholders in healthcare. Nineteen meta-analyses of clinical studies on the management of primary knee osteoarthritis (pkOA) with stem cells, published between January 2020 and July 2021, came to inconsistent conclusions regarding the efficacy of this treatment modality. It is possible that a separate meta-analysis based on an independent, systematic assessment of clinical studies on the management of pkOA with stem cells may reach a different conclusion. (2) Methods: PubMed, Web of Science, and the Cochrane Library were systematically searched for clinical studies and meta-analyses of clinical studies on the management of pkOA with stem cells. All clinical studies and meta-analyses identified were evaluated in detail, as were all sub-analyses included in the meta-analyses. (3) Results: The inconsistent conclusions regarding the efficacy of treating pkOA with stem cells in the 19 assessed meta-analyses were most probably based on substantial differences in literature search strategies among different authors, misconceptions about meta-analyses themselves, and misconceptions about the comparability of different types of stem cells with regard to their safety and regenerative potential. An independent, systematic review of the literature yielded a total of 183 studies, of which 33 were randomized clinical trials, including a total of 6860 patients with pkOA. However, it was not possible to perform a scientifically sound meta-analysis. (4) Conclusions: Clinicians should interpret the results of the 19 assessed meta-analyses of clinical studies on the management of pkOA with stem cells with caution and should be cautious of the conclusions drawn therein. Clinicians and researchers should strive to participate in FDA and/or EMA reviewed and approved clinical trials to provide clinically and statistically valid efficacy.
Keywords: meta-analyses; primary knee osteoarthritis; stem cells; systematic review.
Conflict of interest statement
C.S. is the Advisory Medical Director of InGeneron, Inc. (Houston, TX, USA). C.A. is the Director of Medical and Scientific Affairs of InGeneron. E.U.A. is the Executive Chair of InGeneron. InGeneron had no role in study design, data collection and analysis, or interpretation of the data, and no role in the decision to publish and write this manuscript. No other potential conflicts of interest relevant to this article were reported.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

