Single Cell Effects of Photobiomodulation on Mitochondrial Membrane Potential and Reactive Oxygen Species Production in Human Adipose Mesenchymal Stem Cells
- PMID: 35326423
- PMCID: PMC8946980
- DOI: 10.3390/cells11060972
Single Cell Effects of Photobiomodulation on Mitochondrial Membrane Potential and Reactive Oxygen Species Production in Human Adipose Mesenchymal Stem Cells
Abstract
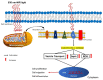
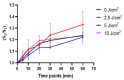
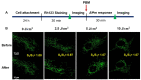
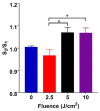
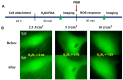
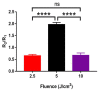
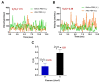
Photobiomodulation (PBM) has recently emerged in cellular therapy as a potent alternative in promoting cell proliferation, migration, and differentiation during tissue regeneration. Herein, a single-cell near-infrared (NIR) laser irradiation system (830 nm) and the image-based approaches were proposed for the investigation of the modulatory effects in mitochondrial membrane potential (ΔΨm), reactive oxygen species (ROS), and vesicle transport in single living human adipose mesenchymal stem cells (hADSCs). The irradiated-hADSCs were then stained with 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA) and Rhodamine 123 (Rh123) to represent the ΔΨm and ROS production, respectively, with irradiation in the range of 2.5-10 (J/cm2), where time series of bright-field images were obtained to determine the vesicle transport phenomena. Present results showed that a fluence of 5 J/cm2 of PBM significantly enhanced the ΔΨm, ROS, and vesicle transport phenomena compared to the control group (0 J/cm2) after 30 min PBM treatment. These findings demonstrate the efficacy and use of PBM in regulating ΔΨm, ROS, and vesicle transport, which have potential in cell proliferation, migration, and differentiation in cell-based therapy.
Keywords: human adipose-derived mesenchymal stem cell; mitochondrial membrane potential; photobiomodulation; reactive oxygen species; vesicle transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Exploring the biphasic dose-response effects of photobiomodulation on the viability, migration, and extracellular vesicle secretion of human adipose mesenchymal stem cells.J Photochem Photobiol B. 2024 Jul;256:112940. doi: 10.1016/j.jphotobiol.2024.112940. Epub 2024 May 14. J Photochem Photobiol B. 2024. PMID: 38776590
-
Alterted Adipogenesis of Human Mesenchymal Stem Cells by Photobiomodulation Using 1064 nm Laser Light.Lasers Surg Med. 2021 Feb;53(2):263-274. doi: 10.1002/lsm.23278. Epub 2020 Jun 3. Lasers Surg Med. 2021. PMID: 32495397
-
Single and consecutive application of near-infrared and green irradiation modulates adipose derived stem cell proliferation and affect differentiation factors.Biochimie. 2022 May;196:225-233. doi: 10.1016/j.biochi.2021.07.009. Epub 2021 Jul 27. Biochimie. 2022. PMID: 34324922
-
Photobiomodulation CME part I: Overview and mechanism of action.J Am Acad Dermatol. 2024 Nov;91(5):793-802. doi: 10.1016/j.jaad.2023.10.073. Epub 2024 Feb 1. J Am Acad Dermatol. 2024. PMID: 38309304 Review.
-
Photobiomodulation of mineralisation in mesenchymal stem cells.Photochem Photobiol Sci. 2021 May;20(5):699-714. doi: 10.1007/s43630-021-00047-5. Epub 2021 May 4. Photochem Photobiol Sci. 2021. PMID: 33945145 Review.
Cited by
-
Use of quantum hyperlight technology in photobiomodulation on stem cells: an experimental in vitro study.Lasers Med Sci. 2025 Feb 15;40(1):96. doi: 10.1007/s10103-025-04358-2. Lasers Med Sci. 2025. PMID: 39954213 Free PMC article.
-
Mitochondrial transplantation: a promising strategy for treating degenerative joint diseases.J Transl Med. 2024 Oct 15;22(1):941. doi: 10.1186/s12967-024-05752-0. J Transl Med. 2024. PMID: 39407249 Free PMC article. Review.
-
The Remodulation of Actin Bundles during the Stimulation of Mitochondria in Adult Human Fibroblasts in Response to Light.Pharmaceutics. 2023 Dec 22;16(1):20. doi: 10.3390/pharmaceutics16010020. Pharmaceutics. 2023. PMID: 38258031 Free PMC article.
-
Photobiomodulation Therapy Improves Repair of Bone Defects Filled by Inorganic Bone Matrix and Fibrin Heterologous Biopolymer.Bioengineering (Basel). 2024 Jan 13;11(1):78. doi: 10.3390/bioengineering11010078. Bioengineering (Basel). 2024. PMID: 38247955 Free PMC article.
-
Osteoblastic differentiation and changes in the redox state in pulp stem cells by laser treatment.Lasers Med Sci. 2024 Mar 6;39(1):87. doi: 10.1007/s10103-024-04016-z. Lasers Med Sci. 2024. PMID: 38443654 Free PMC article.
References
-
- Cheng K.-H., Kuo T.-L., Kuo K.-K., Hsiao C.-C. Human adipose-derived stem cells: Isolation, characterization and current application in regeneration medicine. Genom. Med. Biomark. Health Sci. 2011;3:53–62. doi: 10.1016/j.gmbhs.2011.08.003. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

