Hsp90 in Human Diseases: Molecular Mechanisms to Therapeutic Approaches
- PMID: 35326427
- PMCID: PMC8946885
- DOI: 10.3390/cells11060976
Hsp90 in Human Diseases: Molecular Mechanisms to Therapeutic Approaches
Abstract
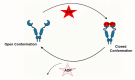
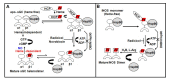
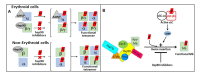
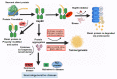
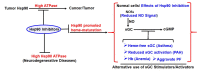
The maturation of hemeprotein dictates that they incorporate heme and become active, but knowledge of this essential cellular process remains incomplete. Studies on chaperon Hsp90 has revealed that it drives functional heme maturation of inducible nitric oxide synthase (iNOS), soluble guanylate cyclase (sGC) hemoglobin (Hb) and myoglobin (Mb) along with other proteins including GAPDH, while globin heme maturations also need an active sGC. In all these cases, Hsp90 interacts with the heme-free or apo-protein and then drives the heme maturation by an ATP dependent process before dissociating from the heme-replete proteins, suggesting that it is a key player in such heme-insertion processes. As the studies on globin maturation also need an active sGC, it connects the globin maturation to the NO-sGC (Nitric oxide-sGC) signal pathway, thereby constituting a novel NO-sGC-Globin axis. Since many aggressive cancer cells make Hbβ/Mb to survive, the dependence of the globin maturation of cancer cells places the NO-sGC signal pathway in a new light for therapeutic intervention. Given the ATPase function of Hsp90 in heme-maturation of client hemeproteins, Hsp90 inhibitors often cause serious side effects and this can encourage the alternate use of sGC activators/stimulators in combination with specific Hsp90 inhibitors for better therapeutic intervention.
Keywords: angiogenesis; heme; heme-free; hemeprotein; metastasis; oncoproteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme.Proc Natl Acad Sci U S A. 2012 Aug 7;109(32):12998-3003. doi: 10.1073/pnas.1205854109. Epub 2012 Jul 25. Proc Natl Acad Sci U S A. 2012. PMID: 22837396 Free PMC article.
-
Myoglobin maturation is driven by the hsp90 chaperone machinery and by soluble guanylyl cyclase.FASEB J. 2019 Sep;33(9):9885-9896. doi: 10.1096/fj.201802793RR. Epub 2019 Jun 6. FASEB J. 2019. PMID: 31170354 Free PMC article.
-
Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content.J Biol Chem. 2014 May 30;289(22):15259-71. doi: 10.1074/jbc.M114.559393. Epub 2014 Apr 14. J Biol Chem. 2014. PMID: 24733395 Free PMC article.
-
Maturation, inactivation, and recovery mechanisms of soluble guanylyl cyclase.J Biol Chem. 2021 Jan-Jun;296:100336. doi: 10.1016/j.jbc.2021.100336. Epub 2021 Jan 26. J Biol Chem. 2021. PMID: 33508317 Free PMC article. Review.
-
New roles for GAPDH, Hsp90, and NO in regulating heme allocation and hemeprotein function in mammals.Biol Chem. 2022 Sep 26;403(11-12):1005-1015. doi: 10.1515/hsz-2022-0197. Print 2022 Nov 25. Biol Chem. 2022. PMID: 36152339 Free PMC article. Review.
Cited by
-
Pharmacokinetics, Dose-Proportionality, and Tolerability of Intravenous Tanespimycin (17-AAG) in Single and Multiple Doses in Dogs: A Potential Novel Treatment for Canine Visceral Leishmaniasis.Pharmaceuticals (Basel). 2024 Jun 11;17(6):767. doi: 10.3390/ph17060767. Pharmaceuticals (Basel). 2024. PMID: 38931434 Free PMC article.
-
Chaperones vs. oxidative stress in the pathobiology of ischemic stroke.Front Mol Neurosci. 2024 Dec 11;17:1513084. doi: 10.3389/fnmol.2024.1513084. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39723236 Free PMC article. Review.
-
The Role of Glucocorticoid Receptor in the Pathophysiology of Pituitary Corticotroph Adenomas.Int J Mol Sci. 2022 Jun 9;23(12):6469. doi: 10.3390/ijms23126469. Int J Mol Sci. 2022. PMID: 35742910 Free PMC article. Review.
-
A novel nanodrug for the sensitization of photothermal chemotherapy for breast cancer in vitro.RSC Adv. 2024 Jul 5;14(30):21292-21299. doi: 10.1039/d4ra01611d. eCollection 2024 Jul 5. RSC Adv. 2024. PMID: 38974230 Free PMC article.
-
The Emerging Role of Heat Shock Factor 1 (HSF1) and Heat Shock Proteins (HSPs) in Ferroptosis.Pathophysiology. 2023 Mar 14;30(1):63-82. doi: 10.3390/pathophysiology30010007. Pathophysiology. 2023. PMID: 36976734 Free PMC article. Review.
References
-
- De Maio A., Santoro M.G., Tanguay R.M., Hightower L.E. Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: A new view of biology, a new society, and a new journal. Cell Stress Chaperones. 2012;17:139–143. doi: 10.1007/s12192-012-0320-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

