T Cells, Interleukin-2 and Systemic Lupus Erythematosus-From Pathophysiology to Therapy
- PMID: 35326431
- PMCID: PMC8946767
- DOI: 10.3390/cells11060980
T Cells, Interleukin-2 and Systemic Lupus Erythematosus-From Pathophysiology to Therapy
Abstract
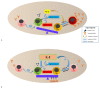
The phenotypic and functional complexities of T cells engender complicated and often confusing concepts as to how T cells ignite, accelerate and brake the inflammatory processes involved in systemic lupus erythematosus (SLE), let alone the plasticity of T cells that takes place under different immunological contexts. Nevertheless, being one of the prime survival factors of T cells, interleukin (IL)-2 plays a potentially critical role in many immunological scenarios during the pathophysiological process of SLE. Here, the pathophysiology of lupus T cells and current, as well as ongoing, therapeutic approaches of SLE that involve low-dose IL-2 administration will be highlighted. The mechanisms of IL-2 deficiency in SLE pathophysiology, the effects of low-dose IL-2 on T cells and restoration of lupus manifestations in murine SLE models, as well as the efficacy and safety of clinical trials that evaluated low-dose IL-2-containing regimens in patients with SLE will be discussed.
Keywords: SLE; T cell; interleukin-2; lupus; regulatory.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

