The Novel Competing Endogenous Long Noncoding RNA SM2 Regulates Gonadotropin Secretion in the Hu Sheep Anterior Pituitary by Targeting the Oar-miR-16b/TGF-β/SMAD2 Signaling Pathway
- PMID: 35326436
- PMCID: PMC8947352
- DOI: 10.3390/cells11060985
The Novel Competing Endogenous Long Noncoding RNA SM2 Regulates Gonadotropin Secretion in the Hu Sheep Anterior Pituitary by Targeting the Oar-miR-16b/TGF-β/SMAD2 Signaling Pathway
Abstract
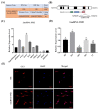
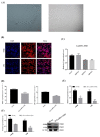
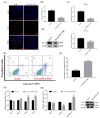
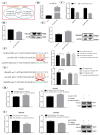
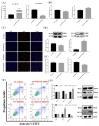
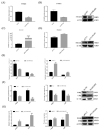
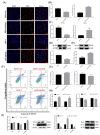
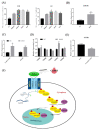
Pituitary gonadotropins play a pivotal role in reproduction. Long noncoding RNAs (lncRNAs) have been identified as important regulators in the hypothalamic−pituitary−ovarian (HPO) axis associated with reproduction. However, the contributions of lncRNAs to pituitary gonadotropin secretion remain largely unknown. Therefore, this work was performed to uncover the functional mechanisms of the novel lncRNA TCONS_00083279 (lncRNA SM2) and its potential targeting pathway oar-miR-16b/TGF-beta/SMAD2, which is associated with gonadotropin secretion in sheep pituitary cells. In the present study, the lncRNA SM2 showed high expression levels in the sheep pituitary gland, and it was located in both the nucleus and the cytoplasm of pituitary cells. lncRNA SM2 knockdown inhibited pituitary cell proliferation and FSH and LH secretion. The function of the lncRNA SM2 was sponged by oar-miR-16b, and this regulated the growth and gonadotropin secretion of pituitary cells by modulating SMAD2, as shown by the dual-luciferase reporter assay. FSH and LH levels were both upregulated by SMAD2 overexpression. Moreover, the levels of the lncRNA SM2, SMAD2 and TGFR1, as well as FSH and LH, in sheep pituitary cells increased significantly under gonadotropin-releasing hormone (GnRH) stimulation (p < 0.05). This work illustrates that the lncRNA SM2 regulates gonadotropin secretion in the Hu sheep anterior pituitary by targeting the oar-miR-16b/TGF-β/SMAD2 signaling pathway, providing a valuable resource for understanding the molecular mechanisms underlying sheep reproduction.
Keywords: TGF-β/SMAD2; gonadotropin; lncRNA SM2; oar-miR-16b; pituitary cells.
Conflict of interest statement
The authors have declared that they have no conflict of interest.
Figures
Similar articles
-
LncRNA-m18as1 competitively binds with miR-18a-5p to regulate follicle-stimulating hormone secretion through the Smad2/3 pathway in rat primary pituitary cells.J Zhejiang Univ Sci B. 2022 Jun 15;23(6):502-514. doi: 10.1631/jzus.B2101052. J Zhejiang Univ Sci B. 2022. PMID: 35686528 Free PMC article.
-
A novel lncRNA LOC105613571 binding with BDNF in pituitary promotes gonadotropin secretion by AKT/ERK-mTOR pathway in sheep associated with prolificacy.Biofactors. 2024 Jan-Feb;50(1):58-73. doi: 10.1002/biof.1990. Epub 2023 Jul 11. Biofactors. 2024. PMID: 37431985
-
Sequencing of the Pituitary Transcriptome after GnRH Treatment Uncovers the Involvement of lncRNA-m23b/miR-23b-3p/CAMK2D in FSH Synthesis and Secretion.Genes (Basel). 2023 Mar 31;14(4):846. doi: 10.3390/genes14040846. Genes (Basel). 2023. PMID: 37107604 Free PMC article.
-
Discovery of new receptors regulating luteinizing hormone and follicle-stimulating hormone secretion by bovine gonadotrophs to explore a new paradigm for mechanisms regulating reproduction.J Reprod Dev. 2020 Aug 20;66(4):291-297. doi: 10.1262/jrd.2020-012. Epub 2020 Apr 6. J Reprod Dev. 2020. PMID: 32249236 Free PMC article. Review.
-
Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation.Biol Reprod. 2013 Feb 14;88(2):35. doi: 10.1095/biolreprod.112.105601. Print 2013 Feb. Biol Reprod. 2013. PMID: 23197164 Review.
Cited by
-
Integrated analysis of microRNA and mRNA interactions regulating fecundity in the ovaries of two distinct sheep breeds.BMC Genomics. 2025 Jul 31;26(1):707. doi: 10.1186/s12864-025-11408-0. BMC Genomics. 2025. PMID: 40745523 Free PMC article.
-
EZH2 Gene Knockdown Inhibits Sheep Pituitary Cell Proliferation via Downregulating the AKT/ERK Signaling Pathway.Int J Mol Sci. 2023 Jun 26;24(13):10656. doi: 10.3390/ijms241310656. Int J Mol Sci. 2023. PMID: 37445833 Free PMC article.
-
Comparative Transcriptomics Identify Key Pituitary Circular RNAs That Participate in Sheep (Ovis aries) Reproduction.Animals (Basel). 2023 Aug 25;13(17):2711. doi: 10.3390/ani13172711. Animals (Basel). 2023. PMID: 37684975 Free PMC article.
-
Oviduct Transcriptomic Reveals the Regulation of mRNAs and lncRNAs Related to Goat Prolificacy in the Luteal Phase.Animals (Basel). 2022 Oct 18;12(20):2823. doi: 10.3390/ani12202823. Animals (Basel). 2022. PMID: 36290212 Free PMC article.
-
Expression profiles of circulating miRNAs in an endangered Piedmontese sheep breed during the estrus cycle.Front Vet Sci. 2024 Nov 5;11:1458463. doi: 10.3389/fvets.2024.1458463. eCollection 2024. Front Vet Sci. 2024. PMID: 39564184 Free PMC article.
References
-
- Farrow P., Simmons J.G., Pozzi E., Diaz-Arteche C., Richmond S., Bray K., Schwartz O., Whittle S. Associations between early life stress and anterior pituitary gland volume development during late childhood. Psychoneuroendocrinology. 2020;122:104868. doi: 10.1016/j.psyneuen.2020.104868. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

