Cooling Uncouples Differentially ROS Production from Respiration and Ca2+ Homeostasis Dynamic in Brain and Heart Mitochondria
- PMID: 35326440
- PMCID: PMC8947173
- DOI: 10.3390/cells11060989
Cooling Uncouples Differentially ROS Production from Respiration and Ca2+ Homeostasis Dynamic in Brain and Heart Mitochondria
Abstract
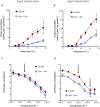
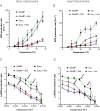
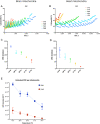
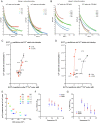
Hypothermia provides an effective neuro and cardio-protection in clinical settings implying ischemia/reperfusion injury (I/R). At the onset of reperfusion, succinate-induced reactive oxygen species (ROS) production, impaired oxidative phosphorylation (OXPHOS), and decreased Ca2+ retention capacity (CRC) concur to mitochondrial damages. We explored the effects of temperature from 6 to 37 °C on OXPHOS, ROS production, and CRC, using isolated mitochondria from mouse brain and heart. Oxygen consumption and ROS production was gradually inhibited when cooling from 37 to 6 °C in brain mitochondria (BM) and heart mitochondria (HM). The decrease in ROS production was gradual in BM but steeper between 31 and 20 °C in HM. In respiring mitochondria, the gradual activation of complex II, in addition of complex I, dramatically enhanced ROS production at all temperatures without modifying respiration, likely because of ubiquinone over-reduction. Finally, CRC values were linearly increased by cooling in both BM and HM. In BM, the Ca2+ uptake rate by the mitochondrial calcium uniporter (MCU) decreased by 2.7-fold between 25 and 37 °C, but decreased by 5.7-fold between 25 and 37 °C in HM. In conclusion, mild cold (25-37 °C) exerts differential inhibitory effects by preventing ROS production, by reverse electron transfer (RET) in BM, and by reducing MCU-mediated Ca2+ uptake rate in BM and HM.
Keywords: Ca2+ retention capacity; MCU; brain mitochondria; cold; heart mitochondria; ischemia/reperfusion; mitochondrial functions; mitochondrial respiration; reactive oxygen species; reverse electron transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tissier R., Chenoune M., Pons S., Zini R., Darbera L., Lidouren F., Ghaleh B., Berdeaux A., Morin D. Mild hypothermia reduces per-ischemic reactive oxygen species production and preserves mitochondrial respiratory complexes. Resuscitation. 2013;84:249–255. doi: 10.1016/j.resuscitation.2012.06.030. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

