Cardiac Cachexia: Unaddressed Aspect in Cancer Patients
- PMID: 35326441
- PMCID: PMC8947289
- DOI: 10.3390/cells11060990
Cardiac Cachexia: Unaddressed Aspect in Cancer Patients
Abstract
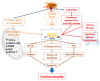
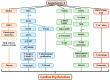
Tumor-derived cachectic factors such as proinflammatory cytokines and neuromodulators not only affect skeletal muscle but also affect other organs, including the heart, in the form of cardiac muscle atrophy, fibrosis, and eventual cardiac dysfunction, resulting in poor quality of life and reduced survival. This article reviews the holistic approaches of existing diagnostic, pathophysiological, and multimodal therapeutic interventions targeting the molecular mechanisms that are responsible for cancer-induced cardiac cachexia. The major drivers of cardiac muscle wasting in cancer patients are autophagy activation by the cytokine-NFkB, TGF β-SMAD3, and angiotensin II-SOCE-STIM-Ca2+ pathways. A lack of diagnostic markers and standard treatment protocols hinder the early diagnosis of cardiac dysfunction and the initiation of preventive measures. However, some novel therapeutic strategies, including the use of Withaferin A, have shown promising results in experimental models, but Withaferin A's effectiveness in human remains to be verified. The combined efforts of cardiologists and oncologists would help to identify cost effective and feasible solutions to restore cardiac function and to increase the survival potential of cancer patients.
Keywords: angiotensin II; autophagy; cancer; cardiac cachexia; chemotherapy; proinflammatory cytokines; withaferin A.
Conflict of interest statement
There exists no conflict of interest among the authors. All authors have read the final version and approved the contents.
Figures



Similar articles
-
Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia.Int J Biochem Cell Biol. 2013 Oct;45(10):2322-32. doi: 10.1016/j.biocel.2013.05.035. Epub 2013 Jun 13. Int J Biochem Cell Biol. 2013. PMID: 23769949 Free PMC article. Review.
-
Molecular Pathways: Cachexia Signaling-A Targeted Approach to Cancer Treatment.Clin Cancer Res. 2016 Aug 15;22(16):3999-4004. doi: 10.1158/1078-0432.CCR-16-0495. Epub 2016 Jun 23. Clin Cancer Res. 2016. PMID: 27340276 Free PMC article. Review.
-
Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions.Biochim Biophys Acta Rev Cancer. 2020 Apr;1873(2):188359. doi: 10.1016/j.bbcan.2020.188359. Epub 2020 Mar 25. Biochim Biophys Acta Rev Cancer. 2020. PMID: 32222610 Free PMC article. Review.
-
Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia.Nat Med. 2016 Jun;22(6):666-71. doi: 10.1038/nm.4093. Epub 2016 May 2. Nat Med. 2016. PMID: 27135739
-
[Cachexia in chronic cardiac disease. Ancient syndrome new idea?].Przegl Lek. 2006;63(3):151-4. Przegl Lek. 2006. PMID: 16967702 Review. Polish.
Cited by
-
Withaferin A Attenuates Muscle Cachexia Induced by Angiotensin II Through Regulating Pathways Activated by Angiotensin II.Cells. 2025 Feb 8;14(4):244. doi: 10.3390/cells14040244. Cells. 2025. PMID: 39996717 Free PMC article.
-
Withaferin A ameliorates ovarian cancer-induced renal damage through the regulation of expression of inflammatory cytokines.J Ovarian Res. 2024 Oct 11;17(1):199. doi: 10.1186/s13048-024-01519-9. J Ovarian Res. 2024. PMID: 39394174 Free PMC article.
-
The pathophysiology of cancer-mediated cardiac cachexia and novel treatment strategies: A narrative review.Cancer Med. 2023 Sep;12(17):17706-17717. doi: 10.1002/cam4.6388. Epub 2023 Sep 1. Cancer Med. 2023. PMID: 37654192 Free PMC article. Review.
-
Highlighting the idea of exerkines in the management of cancer patients with cachexia: novel insights and a critical review.BMC Cancer. 2023 Sep 20;23(1):889. doi: 10.1186/s12885-023-11391-3. BMC Cancer. 2023. PMID: 37730552 Free PMC article. Review.
-
Circulating miRNAs Signature as a Predictor of Cachexia in Chronic Heart Failure: Diagnostic and Prognostic Implications.J Cardiovasc Transl Res. 2025 Jul 10. doi: 10.1007/s12265-025-10658-3. Online ahead of print. J Cardiovasc Transl Res. 2025. PMID: 40637997
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

