Cytochrome c Oxidase Inhibition by ATP Decreases Mitochondrial ROS Production
- PMID: 35326443
- PMCID: PMC8946758
- DOI: 10.3390/cells11060992
Cytochrome c Oxidase Inhibition by ATP Decreases Mitochondrial ROS Production
Abstract
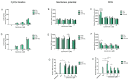
This study addresses the eventual consequence of cytochrome c oxidase (CytOx) inhibition by ATP at high ATP/ADP ratio in isolated rat heart mitochondria. Earlier, it has been demonstrated that the mechanism of allosteric ATP inhibition of CytOx is one of the key regulations of mitochondrial functions. It is relevant that aiming to maintain a high ATP/ADP ratio for the measurement of CytOx activity effectuating the enzymatic inhibition as well as mitochondrial respiration, optimal concentration of mitochondria is critically important. Likewise, only at this concentration, were the differences in ΔΨm and ROS concentrations measured under various conditions significant. Moreover, when CytOx activity was inhibited in the presence of ATP, mitochondrial respiration and ΔΨm both remained static, while the ROS production was markedly decreased. Consubstantial results were found when the electron transport chain was inhibited by antimycin A, letting only CytOx remain functional to support the energy production. This seems to corroborate that the decrease in mitochondrial ROS production is solely the effect of ATP binding to CytOx which results in static respiration as well as membrane potential.
Keywords: ATP; ROS; cytochrome c oxidase; mitochondrial membrane potential.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

