Spermatozoal Mitochondrial Dynamics Markers and Other Functionality-Related Signaling Molecules Exert Circadian-like Response to Repeated Stress of Whole Organism
- PMID: 35326444
- PMCID: PMC8946903
- DOI: 10.3390/cells11060993
Spermatozoal Mitochondrial Dynamics Markers and Other Functionality-Related Signaling Molecules Exert Circadian-like Response to Repeated Stress of Whole Organism
Abstract
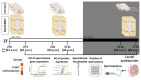
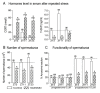
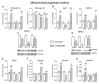
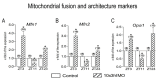
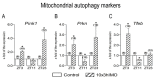
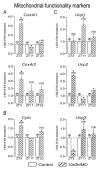
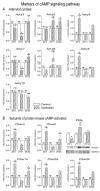
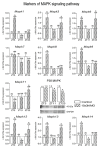
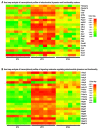
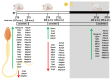
In the search for the possible role of the mitochondrial dynamics markers in spermatozoa adaptation, an in vivo approach was designed to mimic situations in which human populations are exposed to 3 h of repeated psychological stress (the most common stress in human society) at different time points during the day (24 h). The hormones (stress hormone corticosterone and testosterone), the number and the functionality of spermatozoa (response to acrosome-reaction-inducer progesterone), as well as the transcriptional profiles of 22 mitochondrial dynamics and function markers and 22 signaling molecules regulating both mitochondrial dynamics and spermatozoa number and functionality were followed at three time points (ZT3, ZT11, and ZT23). The results show that repeated stress significantly decreased the number and functionality of spermatozoa at all time points. In the same samples, the transcriptional profiles of 91% (20/22) of mitochondrial dynamics and functionality markers and 86% (19/22) of signaling molecules were disturbed after repeated stress. It is important to point out that similar molecular changes in transcriptional profiles were observed at ZT3 and ZT23, but the opposite was observed at ZT11, suggesting the circadian nature of the adaptive response. The results of PCA analysis show the significant separation of repeated stress effects during the inactive/light and active/dark phases of the day, suggesting the circadian timing of molecular adaptations.
Keywords: MAPK signaling markers; cAMP signaling markers; circadian; mitochondrial dynamics and functionality markers; repeated psychological stress response; spermatozoa number and functionality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
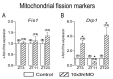


Similar articles
-
Spermatozoa Develop Molecular Machinery to Recover From Acute Stress.Front Endocrinol (Lausanne). 2022 Jul 14;13:896193. doi: 10.3389/fendo.2022.896193. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35909555 Free PMC article.
-
Mitochondrial Dynamics Markers and Related Signaling Molecules Are Important Regulators of Spermatozoa Number and Functionality.Int J Mol Sci. 2021 May 27;22(11):5693. doi: 10.3390/ijms22115693. Int J Mol Sci. 2021. PMID: 34071734 Free PMC article.
-
Reduced spermatozoa functionality during stress is the consequence of adrenergic-mediated disturbance of mitochondrial dynamics markers.Sci Rep. 2020 Oct 8;10(1):16813. doi: 10.1038/s41598-020-73630-y. Sci Rep. 2020. PMID: 33033347 Free PMC article.
-
Nitric oxide is a signaling molecule in spermatozoa.Curr Pharm Des. 2003;9(5):419-25. doi: 10.2174/1381612033391720. Curr Pharm Des. 2003. PMID: 12570819 Review.
-
Sperm function tests and fertility.Int J Androl. 2006 Feb;29(1):69-75; discussion 105-8. doi: 10.1111/j.1365-2605.2005.00630.x. Int J Androl. 2006. PMID: 16466526 Review.
References
-
- Valenti D., Vacca R., Moro L., Atlante A. Mitochondria Can Cross Cell Boundaries: An Overview of the Biological Relevance, Pathophysiological Implications and Therapeutic Perspectives of Intercellular Mitochondrial Transfer. Int. J. Mol. Sci. 2021;22:8312. doi: 10.3390/ijms22158312. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

