Ex Vivo Generation of CAR Macrophages from Hematopoietic Stem and Progenitor Cells for Use in Cancer Therapy
- PMID: 35326445
- PMCID: PMC8947001
- DOI: 10.3390/cells11060994
Ex Vivo Generation of CAR Macrophages from Hematopoietic Stem and Progenitor Cells for Use in Cancer Therapy
Abstract
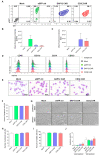
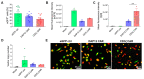
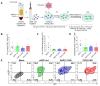
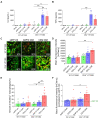
Chimeric antigen receptor (CAR) T-cell therapies have shown impressive results in patients with hematological malignancies; however, little success has been achieved in the treatment of solid tumors. Recently, macrophages (MΦs) were identified as an additional candidate for the CAR approach, and initial proof of concept studies using peripheral blood-derived monocytes showed antigen-redirected activation of CAR MΦs. However, some patients may not be suitable for monocyte-apheresis, and prior cancer treatment regimens may negatively affect immune cell number and functionality. To address this problem, we here introduce primary human hematopoietic stem and progenitor cells (HSPCs) as a cell source to generate functional CAR MΦs ex vivo. Our data showed successful CAR expression in cord blood (CB)-derived HSPCs, with considerable cell expansion during differentiation to CAR MΦs. HSPC-derived MΦs showed typical MΦ morphology, phenotype, and basic anti-bacterial functionality. CAR MΦs targeting the carcinoembryonic antigen (CEA) and containing either a DAP12- or a CD3ζ-derived signaling domain showed antigen redirected activation as they secreted pro-inflammatory cytokines specifically upon contact with CEA+ target cells. In addition, CD3ζ-expressing CAR MΦs exhibited significantly enhanced phagocytosis of CEA+ HT1080 cells. Our data establish human HSPCs as a suitable cell source to generate functional CAR MΦs and further support the use of CAR MΦs in the context of solid tumor therapy.
Keywords: cancer; cancer immunotherapy; chimeric antigen receptors; hematopoietic stem cells; macrophages; phagocytosis; solid tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Scalable generation of functional human iPSC-derived CAR-macrophages that efficiently eradicate CD19-positive leukemia.J Immunother Cancer. 2023 Dec 22;11(12):e007705. doi: 10.1136/jitc-2023-007705. J Immunother Cancer. 2023. PMID: 38135346 Free PMC article.
-
Significantly increased anti-tumor activity of carcinoembryonic antigen-specific chimeric antigen receptor T cells in combination with recombinant human IL-12.Cancer Med. 2019 Aug;8(10):4753-4765. doi: 10.1002/cam4.2361. Epub 2019 Jun 25. Cancer Med. 2019. PMID: 31237116 Free PMC article.
-
CAR T Cells Releasing IL-18 Convert to T-Bethigh FoxO1low Effectors that Exhibit Augmented Activity against Advanced Solid Tumors.Cell Rep. 2017 Dec 12;21(11):3205-3219. doi: 10.1016/j.celrep.2017.11.063. Cell Rep. 2017. PMID: 29241547
-
Engineered CAR-Macrophages as Adoptive Immunotherapies for Solid Tumors.Front Immunol. 2021 Nov 24;12:783305. doi: 10.3389/fimmu.2021.783305. eCollection 2021. Front Immunol. 2021. PMID: 34899748 Free PMC article. Review.
-
Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma.Immunol Rev. 2014 Jan;257(1):83-90. doi: 10.1111/imr.12125. Immunol Rev. 2014. PMID: 24329791 Review.
Cited by
-
The CAR macrophage cells, a novel generation of chimeric antigen-based approach against solid tumors.Biomark Res. 2023 Nov 28;11(1):103. doi: 10.1186/s40364-023-00537-x. Biomark Res. 2023. PMID: 38017494 Free PMC article. Review.
-
A new era of cancer immunotherapy: combining revolutionary technologies for enhanced CAR-M therapy.Mol Cancer. 2024 Jun 1;23(1):117. doi: 10.1186/s12943-024-02032-9. Mol Cancer. 2024. PMID: 38824567 Free PMC article. Review.
-
Unleashing the power of CAR-M therapy in solid tumors: a comprehensive review.Front Immunol. 2025 Jun 12;16:1615760. doi: 10.3389/fimmu.2025.1615760. eCollection 2025. Front Immunol. 2025. PMID: 40574837 Free PMC article. Review.
-
Sophisticated genetically engineered macrophages, CAR-Macs, in hitting the bull's eye for solid cancer immunotherapy approaches.Clin Exp Med. 2023 Nov;23(7):3171-3177. doi: 10.1007/s10238-023-01106-0. Epub 2023 Jun 6. Clin Exp Med. 2023. PMID: 37278931 Review.
-
Advancing CAR-based immunotherapies in solid tumors: CAR- macrophages and neutrophils.Front Immunol. 2023 Nov 28;14:1291619. doi: 10.3389/fimmu.2023.1291619. eCollection 2023. Front Immunol. 2023. PMID: 38090576 Free PMC article. Review.
References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

