Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones
- PMID: 35326451
- PMCID: PMC8947633
- DOI: 10.3390/cells11060997
Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones
Abstract
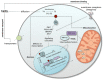
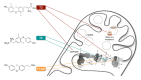
Much is known, but there is also much more to discover, about the actions that thyroid hormones (TH) exert on metabolism. Indeed, despite the fact that thyroid hormones are recognized as one of the most important regulators of metabolic rate, much remains to be clarified on which mechanisms control/regulate these actions. Given their actions on energy metabolism and that mitochondria are the main cellular site where metabolic transformations take place, these organelles have been the subject of extensive investigations. In relatively recent times, new knowledge concerning both thyroid hormones (such as the mechanisms of action, the existence of metabolically active TH derivatives) and the mechanisms of energy transduction such as (among others) dynamics, respiratory chain organization in supercomplexes and cristes organization, have opened new pathways of investigation in the field of the control of energy metabolism and of the mechanisms of action of TH at cellular level. In this review, we highlight the knowledge and approaches about the complex relationship between TH, including some of their derivatives, and the mitochondrial respiratory chain.
Keywords: bioenergetics; iodothyronines; mitochondrial proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mitochondrial Actions of Thyroid Hormone.Compr Physiol. 2016 Sep 15;6(4):1591-1607. doi: 10.1002/cphy.c150019. Compr Physiol. 2016. PMID: 27783852 Review.
-
Thyroid hormone effects on mitochondrial energetics.Thyroid. 2008 Feb;18(2):145-56. doi: 10.1089/thy.2007.0250. Thyroid. 2008. PMID: 18279015 Review.
-
Control of energy metabolism by iodothyronines.J Endocrinol Invest. 2001 Dec;24(11):897-913. doi: 10.1007/BF03343949. J Endocrinol Invest. 2001. PMID: 11817716 Review.
-
Thyroid hormones and mitochondria.Biosci Rep. 2002 Feb;22(1):17-32. doi: 10.1023/a:1016056905347. Biosci Rep. 2002. PMID: 12418548 Review.
-
The key roles of thyroid hormone in mitochondrial regulation, at interface of human health and disease.J Basic Clin Physiol Pharmacol. 2024 Jul 19;35(4-5):231-240. doi: 10.1515/jbcpp-2024-0108. eCollection 2024 Jul 1. J Basic Clin Physiol Pharmacol. 2024. PMID: 39023546 Free PMC article. Review.
Cited by
-
Application of Human Plasma Targeted Lipidomics and Analysis of Toxic Elements to Capture the Metabolic Complexities of Hypothyroidism.Molecules. 2024 Oct 31;29(21):5169. doi: 10.3390/molecules29215169. Molecules. 2024. PMID: 39519809 Free PMC article.
-
Structural and Dynamic Features of Liver Mitochondria and Mitophagy in Rats with Hyperthyroidism.Int J Mol Sci. 2022 Nov 18;23(22):14327. doi: 10.3390/ijms232214327. Int J Mol Sci. 2022. PMID: 36430802 Free PMC article.
-
Optimized Mass Spectrometry Detection of Thyroid Hormones and Polar Metabolites in Rodent Cerebrospinal Fluid.Metabolites. 2024 Jan 23;14(2):79. doi: 10.3390/metabo14020079. Metabolites. 2024. PMID: 38392972 Free PMC article.
-
Extranuclear effects of thyroid hormones and analogs during development: An old mechanism with emerging roles.Front Endocrinol (Lausanne). 2022 Sep 23;13:961744. doi: 10.3389/fendo.2022.961744. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36213288 Free PMC article. Review.
-
Effects of the Long-Term Administration of Uridine on the Functioning of Rat Liver Mitochondria in Hyperthyroidism.Int J Mol Sci. 2023 Nov 24;24(23):16730. doi: 10.3390/ijms242316730. Int J Mol Sci. 2023. PMID: 38069053 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

