Vogt-Koyanagi-Harada Disease Exacerbation Associated with COVID-19 Vaccine
- PMID: 35326462
- PMCID: PMC8947156
- DOI: 10.3390/cells11061012
Vogt-Koyanagi-Harada Disease Exacerbation Associated with COVID-19 Vaccine
Abstract
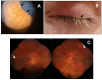
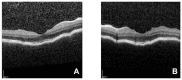
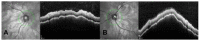
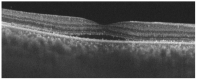
We describe a case of Vogt-Koyanagi-Harada (VKH) disease exacerbation after COVID-19 vaccination. A 46-year-old woman presented with a bilateral granulomatous uveitis 2 days after the first dose of COVID-19 mRNA vaccine (Comirnaty, Pfizer-BioNTech), and was diagnosed with a complete Vogt-Koyanagi-Harada (VKH) disease 4 days after receiving the second dose of the vaccine. Three weeks before the first dose, she had been consulted for blurred vision and mild headaches. The case resolved with high dose intravenous corticosteroids, followed by oral prednisone. The close temporal relationship between the COVID-19 vaccine doses and the worsening of VKH symptoms strongly suggests COVID-19 vaccination as the trigger of its exacerbation.
Keywords: COVID-19 vaccine; VKH disease; uveitis; vaccine-associated autoimmunity; vitiligo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Vitiligo as a First Sign of Vogt-Koyanagi-Harada Disease.Acta Dermatovenerol Croat. 2023 Dec;31(4):229-231. Acta Dermatovenerol Croat. 2023. PMID: 38651852
-
De Novo Vogt-Koyanagi-Harada Disease following Covid-19 Vaccine: A Case Report and Literature Overview.Ocul Immunol Inflamm. 2022 Jul;30(5):1292-1295. doi: 10.1080/09273948.2022.2028291. Epub 2022 Feb 3. Ocul Immunol Inflamm. 2022. PMID: 35113742 Review.
-
Vogt-Koyanagi-Harada Disease Associated with COVID-19 mRNA Vaccine.Ocul Immunol Inflamm. 2021 Aug 18;29(6):1212-1215. doi: 10.1080/09273948.2021.1974492. Epub 2021 Sep 10. Ocul Immunol Inflamm. 2021. PMID: 34505819
-
Vogt-Koyanagi-Harada Disease following mRNA-1273 (Moderna) COVID-19 Vaccination.Ocul Immunol Inflamm. 2022 Jul;30(5):1250-1254. doi: 10.1080/09273948.2022.2053547. Epub 2022 Apr 11. Ocul Immunol Inflamm. 2022. PMID: 35404752
-
Vogt-Koyanagi-Harada disease after SARS-CoV-2 infection: Case report and literature review.Immun Inflamm Dis. 2024 Apr;12(4):e1250. doi: 10.1002/iid3.1250. Immun Inflamm Dis. 2024. PMID: 38661242 Free PMC article. Review.
Cited by
-
The Characteristics of COVID-19 Vaccine-Associated Uveitis: A Summative Systematic Review.Vaccines (Basel). 2022 Dec 28;11(1):69. doi: 10.3390/vaccines11010069. Vaccines (Basel). 2022. PMID: 36679914 Free PMC article. Review.
-
Correction.Hum Vaccin Immunother. 2023 Aug;19(2):2259160. doi: 10.1080/21645515.2023.2259160. Epub 2023 Sep 21. Hum Vaccin Immunother. 2023. PMID: 37732617 Free PMC article. No abstract available.
-
Challenges in posterior uveitis-tips and tricks for the retina specialist.J Ophthalmic Inflamm Infect. 2023 Aug 17;13(1):35. doi: 10.1186/s12348-023-00342-5. J Ophthalmic Inflamm Infect. 2023. PMID: 37589912 Free PMC article. Review.
-
Bibliometric analysis of the Vogt‒Koyanagi‒Harada disease literature.Int Ophthalmol. 2023 Nov;43(11):4137-4150. doi: 10.1007/s10792-023-02815-x. Epub 2023 Aug 8. Int Ophthalmol. 2023. PMID: 37552428 Free PMC article.
-
Vogt-Koyanagi-Harada Syndrome Following COVID-19 mRNA Vaccination: Th2 to Th1 Transition-related Molecular Machinery.Acta Derm Venereol. 2024 May 13;104:adv21502. doi: 10.2340/actadv.v104.21502. Acta Derm Venereol. 2024. PMID: 38738771 Free PMC article. No abstract available.
References
-
- Boyarsky B.J., Ruddy J.A., Connolly C.M., Ou M.T., Werbel W.A., Garonzik-Wang J.M., Segev D.L., Paik J.J. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80:1098–1099. doi: 10.1136/annrheumdis-2021-220289. - DOI - PMC - PubMed
-
- Connolly C.M., Ruddy J.A., Boyarsky B.J., Avery R.K., Werbel W.A., Segev D.L., Garonzik-Wang J., Paik J.J. Safety ofthefirstdose ofmRNA SARS-CoV-2vaccines inpatients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80:1100–1101. doi: 10.1136/annrheumdis-2021-220231. - DOI - PMC - PubMed
-
- Geisen U.M., Berner D.K., Tran F., Sumbul M., Vullriede L., Ciripoi M., Reid H.M., Schaffarzyk A., Longardt A.C., Franzenburg J., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. - DOI - PMC - PubMed
-
- Gargano J.W., Wallace M., Hadler S.C., Langley G., Su J.R., Oster M.E., Broder K.R., Gee J., Weintraub E., Shimabukuro T., et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. Morb. Mortal. Wkly. Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

