Retrospective Analysis of Autologous Chondrocyte-Based Cytotherapy Production for Clinical Use: GMP Process-Based Manufacturing Optimization in a Swiss University Hospital
- PMID: 35326468
- PMCID: PMC8947208
- DOI: 10.3390/cells11061016
Retrospective Analysis of Autologous Chondrocyte-Based Cytotherapy Production for Clinical Use: GMP Process-Based Manufacturing Optimization in a Swiss University Hospital
Abstract
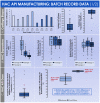
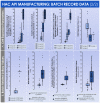
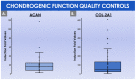
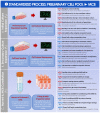
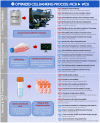
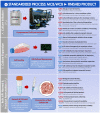
Cultured autologous human articular chondrocyte (HAC) implantation has been extensively investigated for safe and effective promotion of structural and functional restoration of knee cartilage lesions. HAC-based cytotherapeutic products for clinical use must be manufactured under an appropriate quality assurance system and follow good manufacturing practices (GMP). A prospective clinical trial is ongoing in the Lausanne University Hospital, where the HAC manufacturing processes have been implemented internally. Following laboratory development and in-house GMP transposition of HAC cell therapy manufacturing, a total of 47 patients have been treated to date. The main aim of the present study was to retrospectively analyze the available manufacturing records of the produced HAC-based cytotherapeutic products, outlining the inter-individual variability existing among the 47 patients regarding standardized transplant product preparation. These data were used to ameliorate and to ensure the continued high quality of cytotherapeutic care in view of further clinical investigations, based on the synthetic analyses of existing GMP records. Therefore, a renewed risk analysis-based process definition was performed, with specific focus set on process parameters, controls, targets, and acceptance criteria. Overall, high importance of the interdisciplinary collaboration and of the manufacturing process robustness was underlined, considering the high variability (i.e., quantitative, functional) existing between the treated patients and between the derived primary HAC cell types.
Keywords: ATMP; GMP manufacturing; autologous chondrocyte implantation; cartilage defect; cell therapy; optimization; process controls; production process; standardized transplant product; technical workflows.
Conflict of interest statement
Author A.L. was employed by LAM Biotechnologies S.A. during the performance of this work. The remaining authors declare no conflict of interest.
Figures




References
-
- Labedz-Maslowska A., Szkaradek A., Mirzwinski T., Madeja Z., Zuba-Surma E. Processing and ex vivo expansion of adipose tissue-derived mesenchymal stem/stromal cells for the development of an advanced therapy medicinal product for use in humans. Cells. 2021;10:1908. doi: 10.3390/cells10081908. - DOI - PMC - PubMed
-
- Pearce K.F., Hildebrandt M., Greinix H., Scheding S., Koehl U., Worel N., Apperley J., Edinger M., Hauser A., Mischak-Weissinger E., et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy. 2014;16:289–297. doi: 10.1016/j.jcyt.2013.08.003. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

