Clinical Trials Using Mesenchymal Stem Cells for Spinal Cord Injury: Challenges in Generating Evidence
- PMID: 35326470
- PMCID: PMC8946989
- DOI: 10.3390/cells11061019
Clinical Trials Using Mesenchymal Stem Cells for Spinal Cord Injury: Challenges in Generating Evidence
Abstract
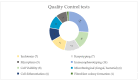
Spinal cord injury (SCI) remains an important public health problem which often causes permanent loss of muscle strength, sensation, and function below the site of the injury, generating physical, psychological, and social impacts throughout the lives of the affected individuals, since there are no effective treatments available. The use of stem cells has been investigated as a therapeutic approach for the treatment of SCI. Although a significant number of studies have been conducted in pre-clinical and clinical settings, so far there is no established cell therapy for the treatment of SCI. One aspect that makes it difficult to evaluate the efficacy is the heterogeneity of experimental designs in the clinical trials that have been published. Cell transplantation methods vary widely among the trials, and there are still no standardized protocols or recommendations for the therapeutic use of stem cells in SCI. Among the different cell types, mesenchymal stem/stromal cells (MSCs) are the most frequently tested in clinical trials for SCI treatment. This study reviews the clinical applications of MSCs for SCI, focusing on the critical analysis of 17 clinical trials published thus far, with emphasis on their design and quality. Moreover, it highlights the need for more evidence-based studies designed as randomized controlled trials and potential challenges to be addressed in context of stem cell therapies for SCI.
Keywords: clinical trial; mesenchymal stem cells; mesenchymal stromal cells; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






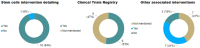



References
-
- Brasil . Diretrizes de Atenção à Pessoa com Lesão Medular. Ministério da Saúde Secretaria de Atenção à Saúde Departamento de Ações Programáticas Estratégicas; Brasilia, Brazil: 2015.
-
- Bombardier C.H., Azuero C.B., Fann J.R., Kautz D.D., Richards J.S., Sabharwal S. Management of Mental Health Disorders, Substance Use Disorders, and Suicide in Adults with Spinal Cord Injury: Clinical Practice Guideline for Healthcare Providers. Top. Spinal Cord Inj. Rehabil. 2021;27:152–224. - PMC - PubMed
-
- Janahú L.T.A., Neves L.M.T., Silva M.C., Oliveira I.S. Trauma raquimedular: Perfil epidemiológico dos pacientes atendidos no Pronto Socorro Municipal Mário Pinotti nos anos de 2003 à 2005. Fisioter. Ser. 2009;4:246–249.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

